Bacterial Contamination of Selected Fruits, Fresh Juice Contact Surfaces and Processor’s Hands: Potential Risk for Consumers’ Health in Uganda
Article Information
Phoebe P Kaddumukasa1,2*, Samuel M Imathiu2, Julius M Mathara2, Jesca L Nakavuma3
1Department of Food Technology, Faculty of Science, Kyambogo University, Kampala, Uganda
2Department of Food science and Technology, School of Food and Nutrition sciences, College of Agriculture, Natural Resources and Environment, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
3Department of Microbiology, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
*Corresponding Author: Phoebe P Kaddumukasa, Department of Food Technology, Faculty of Science, Kyambogo University, Kampala, Uganda
Received: 29 July 2019; Accepted: 14 August 2019; Published: 30 August 2019
Citation:
Phoebe P Kaddumukasa, Samuel M Imathiu, Julius M Mathara, Jesca L Nakavuma. Bacterial Contamination of Selected Fruits, Fresh Juice Contact Surfaces and Processor’s Hands: Potential Risk for Consumers’ Health in Uganda. Journal of Food Science and Nutrition Research 2 (2019): 199-213.
Share at FacebookAbstract
A cross sectional study to assess bacteriological safety of 60 fruits, 85 juice contact surfaces and 30 hands was carried out in Kampala, Uganda. Sampling was done according to ISO 18593. Mean aerobic plate counts of 8.3, 8.6 and 8.5 log10CFU/cm² on passion, mango and pineapple fruit surfaces respectively, were obtained. Juice dispensers, refrigerators and hands had mean aerobic plate counts of 5.6, 5.9 and 7 log10 CFU/cm² respectively. Mean coliform counts of 4, 3.9 and 3.7 log10 CFU/cm² were observed for dispenser, refrigerator and hands respectively. Mean S. aureus count of 5 log10 CFU/cm2 and range from no detection to 6.8 log10 CFU/cm2 was observed for dispenser surfaces. Thirty-eight (56.7%), n=67 refrigerators were contaminated with S. aureus above the detection limit. Eighteen (60%), of 30 hand samples were contaminated with S. aureus above the detection limit. Staphylococcus aureus was the most prevalent pathogen while Salmonella and Listeria spp were absent from all samples. One (6.7%) out of 15 coagulase positive isolates was positive for the mecA gene. These findings show that fruit, fresh juice contact surfaces and hands can be potential vehicles through which bacterial contamination could occur in fresh juices. There is therefore great need to emphasize stringent hygiene and use of good manufacturing practices to ensure production of microbiologically safe products.
Keywords
Bacterial contamination, Food contact surfaces, Fruit juice, Microbial quality, Refrigerators
Bacterial contamination articles, Food contact surfaces articles, Fruit juice articles, Microbial quality articles, Refrigerators articles
Article Details
1. Introduction
Vending of fresh unpasteurized juices is widely practiced in many developing countries, particularly in urban areas [1]. The juices are made from a variety of fruits grown within Uganda while some are imported from the neighboring countries within the East African region. The marketing of passion fruit is well developed with imports coming into Uganda from Kenya and Rwanda. A small proportion of fruits from Mbale in Uganda are imported into Kenya [2]. Uganda receives 80% of its passion fruit from Kenya. Uganda produces only 0.35% of the pineapples in East Africa with 40% of these fruits being exported into the regional markets of Democratic Republic of Congo, Rwanda and Kenya [2]. Eighty percent and 19% of pineapples in East Africa are produced by Kenya and Tanzania respectively [2]. It is from some of these fruits grown and imported into Uganda that the vended fresh fruit juices are made. Juice vending requires low capital investment and attracts many rural immigrants to cities in developing countries to make a living [3].
The juices made are served in restaurants, cafes, fast food establishments and in busy places such as markets, roadside shops and bus terminals which provide a range of fresh products to consumers. These juices are in great demand for their perceived health, nutritional benefits and availability at reasonable prices [4]. Juices served in permanent structures are offered in better hygienic conditions than those in make-shift structures [5]. The microbiological quality of street-vended juices, just like most street foods, is largely questionable because of the poor hygienic environment associated with their processing [6]. Consumers of these juices are therefore at the mercy of vendors, who most often have low education in hygienic practices and are more interested in making a living than in the safety of the end users. Fresh unpasteurized juices clearly are products worthy of concern in causing potential food safety risk [7].
Consumption of fresh juices has resulted in foodborne illnesses from among other pathogenic microorganisms Escherichia coli, Salmonella, Shigella and Staphylococcus aureus [8]. Illnesses caused by fresh juices are reported to arise from contamination from raw materials, equipment, processing conditions, improper handling, unpotable water as well as increased length of storage at room temperature [9]. Furthermore, the exposure of these juices to airborne dust and unhygienic conditions such as dirty waste water and garbage near the food establishments allows vectors like houseflies to transfer microorganisms to the finished products [1, 8]. Data in the literature suggests that about 50% of the microbial foodborne illnesses are caused by inappropriate food storage mainly due to ineffective chill storage and refrigeration management [5, 10]. The quality and safety of fresh unpasteurized juices for consumers’ health is thus important. Information on the hygiene of foods, food contact surfaces and processors’ hands is lacking within the Ugandan Standards.
Hygienic requirements for food establishments are available in the East African Standards, however information of surface contamination of foods, food contact surfaces and processors’ hands is lacking. This may be the first study to report such findings within this region. Good manufacturing practices and personal hygiene, which may be lacking during processing, need to be emphasized in order to produce wholesome products. The aim of the study was to assess the bacterial quality of selected fruits, fresh juice contact surfaces and processors hands, and to gain an understanding of their contamination status.
2. Materials and Methods
2.1 Study area
The study was conducted in Kampala city, Uganda (00° 19’ N, 32° 35”E) from November 2014 to October 2015 using a cross sectional research design. Three out of the five divisions of the city were selected based on the numerous retail outlets supplying fresh juices and the large population supporting these outlets. The selected divisions included; Kampala central, Kawempe and Nakawa (Figure 1).
2.2 Sample collection and processing
Samples used included swabs from the most common fruits used for juice processing that is, passion fruits, pineapples, and mangoes, juice dispensing units, refrigerators and processors hands. The swab method according to ISO 18593 [11] with modifications was used. A sterile template measuring 2.5 × 2.5 cm² was used for fruits and juice vendors’ hands after obtaining permission with informed consent. For each of the selected surfaces, proper labeling of the swab tube with sufficient detail (name of the sample, date of collection, market from which the sample was purchased) was done to enable easy identification of the sample after analysis. The swab stick was removed from the sterile wrapping followed by moistening of the tip in 10 mL of 0.1% sterile buffered peptone water (BPW) (Oxoid, Hampshire, UK) in a resuspension tube. The moistened tip was pressed against the inside wall of the tube to remove excess fluid. The tip of the swab was pressed onto the selected surface and streaked in two directions at right angles within the template whilst rotating the swab stick between thumb and forefinger. The used swab was placed back in the tube and aseptically cut off from the stick. Samples were placed in an ice box and transported to the laboratory in the College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB), Makerere University, Kampala. For the dispensing units and refrigerators, a sterile sponge template of 100 cm² was used.
Briefly, ten sterile test tubes were dispensed with 9 ml of sterilized BPW (Oxoid Ltd, UK) for each sample. Ten fold serial dilutions were prepared in BPW from 10-1 to 10-10 and vortexed for proper mixing to form the stock for each sample. The stocks were then used for bacteriological analysis of all samples.
2.3 Bacteriological analysis of swabbed surfaces.
For bacteriological analysis, the aerobic mesophiles, coliforms, E. coli, S. aureus, Salmonella and Listeria spp were done. Aerobic mesophilic plate counts (APCs) were conducted according to [12]. After mixing each tube with the dilution, duplicate dilutions of 0.1 mL of mixture was surface spread onto duplicate sterile plate count agar (PCA) (Oxoid Ltd, Basingstoke Hants, England), and immediately placed in an incubator set at 37°C for 24 hours. Plates were observed for the presence of discrete colonies and the actual numbers of bacteria were estimated as colony forming unit per mL (CFU/ml). Total coliform counts (TCC) were estimated using violet red bile agar following the described protocol [13]. A 0.1 mL of sample dilution was transferred onto culture plate containing violet red bile (VRBA) agar (Oxoid, UK) using a sterile pipette. Duplicate dilutions ranging from 10?¹ to 10?? were used for each sample and were incubated at 35°C for 48 hr after which readings were taken. The presence of coliforms was indicated by colonies with a reddish maroon color. Escherichia coli was enumerated according to the protocol described [13]. For E. coli counts, MacConkey agar (MAC) (Himedia, India) plates were surface spread, inoculated in duplicate with 0.1 mL of resuspension liquid for each sample. Incubation was done at 44°C for 24 hours.
Presence of Escherichia coli was indicated by colonies with a purple-red color of approximately 0.5 mm in diameter. Staphylococcus aureus counts were performed using mannitol salt agar (MSA) (Chapman medium, Eur. Pharm) using the described method [14]. Counts for S. aureus were surface spread in duplicate using inoculations of 0.1 mL on MSA plates and incubated at 37°C for 48 hours. Staphylococcus aureus colonies were identified by a golden yellow color. Presence or absence of Salmonella spp. was determined using the method described [15]. A 1 mL mixture was transferred into 10 mL selenite cystine enrichment broth (SCB) and another 1 mL mixture into 10 mL of Tetrathionate broth, vortexed and incubated at 35 ± 2°C. A volume of 0.1 mL was transferred into 10 mL of Rapapport- Vassiliadis medium (Himedia, India), vortexed and incubated at 42°C for 24 hr to allow for selective enrichment of Salmonella spp. The cultures were streaked on xylose lysine desoxycholate (XLD) agar and further incubated at 35°C for 24 hr. The plates were examined for typical Salmonella colonies characterized by a pink colour with or without black centers. Listeria monocytogenes was isolated using the protocol described [16] as follows; 25 mL of sample was pipetted into 225 mL of Fraser Listeria enrichment broth (Oxoid, UK) and mixed in a stomacher bag for 2 minutes. Duplicate serial dilutions ranging from 10?¹ to 10?? were cultured. This was followed by incubation of 1 mL of the enrichment culture on Listeria Fraser medium (Oxoid, UK) agar for 48 hours at 30°C. Presence of L. monocytogenes was indicated by the presence of round 1 mm colonies surrounded by a black zone.
2.3.1 Counting of colonies obtained from the swabs: Counting of the colonies was calculated per centimeter of initial swab surfaces as described in the international standard [11]. The number of CFU per square centimeter of surface, Ns was calculated using the formula;
Ns = N × F/A
Where N is the number of CFU in 1 mL of dilution liquid, F is the amount in milliliters of dilution fluid in the tube, A is the surface investigated in square centimeters and D is the reciprocal of the dilution used.
2.3.2 Biochemical identification of isolates: Various biochemical tests were used for the identified microorganisms including; indole test, methyl red test, Voges Proskauer test, citrate utilization test, catalase test, oxidase test, coagulase test and the Gram stain technique.
2.4 Detection of mecA gene by polymerase chain reaction
DNA extraction was carried out using a QIAGEN kit (GmbH, Hilden, Germany) following manufacturers’ instructions to identify the presence or absence of mecA gene in the S. aureus isolates. Five milliliters of overnight bacterial culture was centrifuged at 8,000 rpm at room temperature to obtain a pellet. The pellet of bacterial cells was re-suspended in 250 µL of P1 buffer and transferred to a microcentrifuge tube (HERMLE, Labartechnik, GMBH). Two hundred and fifty microlitres of P2 buffer was added to the pellet and the contents were mixed thoroughly by inversion of the tube 5 times until the solution was clear. This was also done for N3 buffer when 350 µL were added. The contents were centrifuged for 10 minutes in a microcentrifuge at 13,000 rpm. The supernatant was decanted into a spin column and centrifuged for 60 seconds which allowed the flow through to pass into the spin column. The spin column was washed by adding 500 µL of PB buffer followed by centrifugation for 60 seconds to allow flow through to pass out of the spin column. The content in the spin column was washed by adding 750 µL of PE buffer, centrifuged for 60 seconds and the flow through discarded. The content was centrifuged again to remove residual wash buffer. The column was then placed in a new clean 1.5 mL microcentrifuge tube and the DNA eluted by adding 50 µL of EB buffer, left to stand for 1 minute and centrifuged for 1 minute.
DNA fragments carrying transposon/ chromosome junction sequences for methicillin resistance were amplified by PCR using the following primer pairs; mecA-F (ACGAGTAGATGCTCAATATAA) and mecA-R (CTTAGTTCTTTAGAGATTGA) [17]. Thirteen microlitres were prepared for mecA amplification consisting of 6.25 µL of 2 × Phusion taq DNA polymerase, double distilled water (5.4 µL), forward primer (0.2 µL), reverse primer (0.2 µL), template (0.5 µL) and PCR water. The thermocycling conditions consisted of initial denaturation at 95°C for 5 minutes, 37 cycles at 95°C for 30 seconds, annealing at 55°C for 30 seconds and primer extension of 72°C for 60 seconds, with a final extension at 72°C for 10 minutes.
2.5 Statistical analysis
Bacterial counts were transformed into logs before statistical analysis. The data were analyzed with ANOVA Graph Pad Prism version 7.0 (Graph pad software Inc, La Jolla, Ca). Means were compared using the Tukey test. The level of significance was set at α=0.05.
3. Results
3.1 Bacteriological contamination of swabbed fresh fruit surfaces
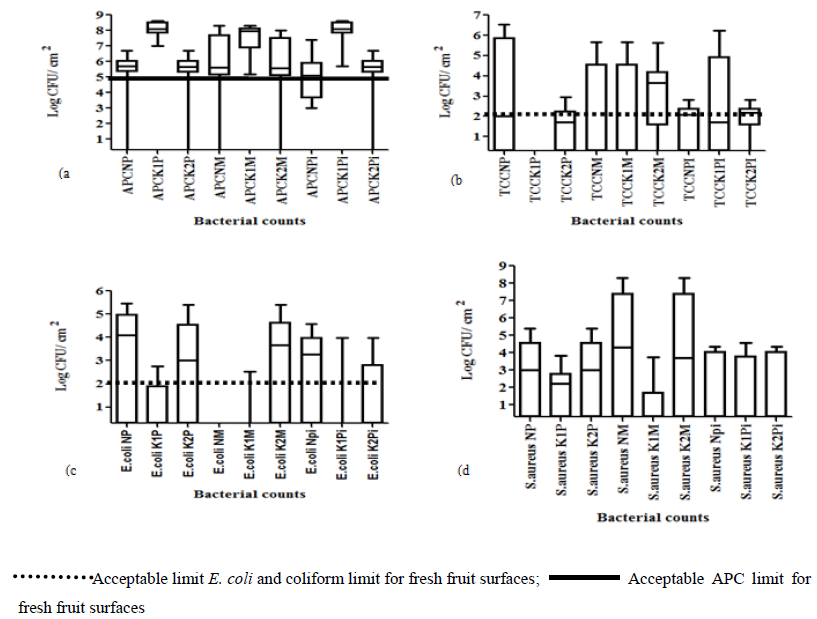
Bacteriological analysis of fresh fruit surfaces revealed the presence of aerobic mesophiles, coliforms, E. coli and S. aureus on the swabbed samples (Figure 2).
3.1.1 Aerobic plate counts: Overall, APCs for swabbed passion fruit, mango, and pineapple samples in all divisions were above 5 log10 CFU/cm². Significantly high (p<0.05) APC means of 8.3 log10 CFU/cm², 8.6 log10 CFU/cm², 8.5 log10 CFU/cm² were observed in Kampala central for passion, mango, and pineapple fruit samples than those from Kawempe and Nakawa (Figure 2a). Lowest APC contamination for fruit samples was observed in Kawempe with no detection for 2 mango samples and 1 sample each for both passion and pineapple samples (Figure 2a). Mango samples were not significantly different (p>0.05) in APCs for all three divisions. Significant differences (p<0.05) in APCs for pineapple samples were observed between Kampala central and Kawempe.
3.1.2 Total coliform counts: Highest coliform count for passion fruit samples was observed in Nakawa division with a mean of 5.7 log10 CFU/cm² and a range 0-6.5 log10 CFU/ cm² (Figure 2b). Lowest coliform count ranging from 0-3 log10 CFU/cm² was observed in Kampala central with passion fruit samples showing no detection for coliforms. Kawempe and Nakawa both had a range from no detection to 6.5 log10 CFU/cm². Non significant (p>0.05) coliform ranges of 0 - 5.7 log10 CFU/ cm², 0 - 5.7 log10 CFU/cm², 0 - 5.6 log10 CFU/ cm² were observed for mango samples in Nakawa, Kampala central, and Kawempe divisions, respectively. The highest number of coliforms of (6.2 log10 CFU/cm²) in pineapple samples was observed in Kampala central while low counts were obtained from Kawempe and Nakawa divisions. Coliforms on pineapple samples in Kampala central were significantly higher (p<0.05) than those from Nakawa and Kawempe.
3.1.3 Escherichia coli counts: Escherichia coli above the detection limit were found on all passion fruit samples. Ranges of 0-5.5 log10 CFU/ cm², 0-2.75 log10 CFU/cm², were observed in Kampala central and Nakawa, respectively (Figure 2c). Kampala central passion fruit samples had significantly lower (p<0.05) E. coli counts than for samples from Nakawa and Kawempe. Mango samples in Kawempe had E. coli counts ranging from no detection to 5.4 log10 CFU/cm², which were significantly higher (p<0.05) than those from Nakawa with no detection and Kampala central with a range of 0-2.53 log10 CFU/cm². Non significant differences (p>0.05) in E. coli counts were observed for pineapple samples with ranges of 0-4.6 log10 CFU/cm², 0-4 log10 CFU/cm², 0-4.3 log10 CFU/cm², for Nakawa, Kampala central, and Kawempe, respectively (Figure 2c).
3.1.4 Staphylococcus aureus counts: Passion fruit samples in Kampala central showed a significantly lower (p<0.05) S. aureus mean count of 2.94 log10 CFU/cm² with a range from no detection to 3.8 log10 CFU/cm². Non significant differences (p>0.05) in S. aureus counts were observed for Kawempe and Nakawa both with a mean of 4.6 log10 CFU/cm² and range from 0-5.4 log10 CFU/cm² (Figure 2d). Mango samples in Nakawa and Kawempe had the same mean S. aureus count and range of 7.5 log10 CFU/cm², 8.3 log10 CFU/cm², respectively. Non significantly different (p>0.05) S. aureus mean counts of (3.6, 3.7, 3.6) log10 CFU/cm² and ranges of (0-5.4, 0-4.6 0-4.3) log10 CFU/cm², were observed for pineapple samples in Nakawa, Kampala central, and Kawempe, respectively (Figure 2d).
3.2 Bacterial contamination of fresh juice contact surfaces and processors’ hands
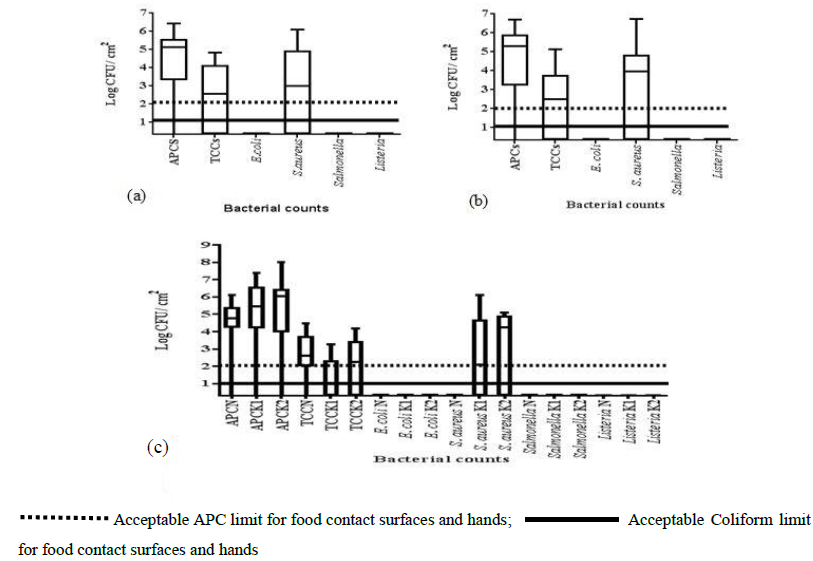
Bacteriological analysis of sample surfaces indicated the presence of aerobic mesophiles, coliforms, and S. aureus while E. coli, Salmonella and Listeria spp were absent (Figure 3).
3.2.1 Bacterial contamination of juice dispensers: Aerobic plate counts (APCs) of juice contact surfaces showed high prevalence above 2 log10 CFU/cm². Seventeen out of the 18 (94.4%) dispensers in this study had aerobic mesophiles above 2 log10 CFU/cm² with a mean aerobic count of 5.6 log10 CFU/cm² (Figure 3a). Dispenser sample range was from 0-6.1 log10 CFU/cm². Coliforms were not detected in eight out of 18 dispensers (44.5%) while 10 (55.5%) dispensers were contaminated above the no detection limit. A mean coliform count of 4 log10 CFU/ cm² with a range from 0- 4.8 log10 CFU/cm² was observed for the dispensers. A mean S. aureus count of 5.04 log10 CFU/cm² with a range from no detection to 6.8 log10 CFU/cm² was obtained from the swabbed dispenser surfaces.
3.2.2 Bacterial contamination of refrigerator surfaces: Aerobic plate counts were not detected in five out of 67 (7.5%) samples while 62 out of 67 (92.5%) refrigerator surfaces had APCs above 2 log10 CFU/cm² with a mean of 5.9 log10 CFU/cm² and range from 0-6.7 log10 CFU/ cm² (Figure 3b). Coliforms were not detected in twenty-six (39%) (n=67) refrigerators while 41 (61%) presented coliforms above the detection limits with a mean of 3.9 log10 CFU/ cm² and range from no detection to 6.1 log10 CFU/cm² (Figure 2b). Non significant differences (p>0.05) in coliforms were observed for the refrigerators with a range from no detection to 5.85 log10 CFU/ cm². Twenty-nine of 67 (43.3%) samples showed no detection for S. aureus while 38 (56.7%) refrigerator surfaces were above the detection limit for food contact surfaces.
3.2.3 Bacterial contamination of vendors’ hands: Aerobic mesophiles on 26 out of 30 (86.7%) hand samples were obtained in Kampala. Aerobic mesophile ranged from no detection to 8 log10 CFU/cm² with the highest mean of 7 log10 CFU/ cm² in Kawempe and the lowest mean of 5.4 log10 CFU/cm² in Nakawa. However, Nakawa hand samples were significantly lower (p<0.05) in APCs than those from Kampala central and Kawempe (Figure 3c). Coliforms were detected in (60%) (n=30) hand samples above the detection limit while no detection was observed for 40% of the samples (Figure 3c). Highest (9/10) coliform detection in hand samples was observed in Nakawa with a mean of 3.7 log10 CFU/ cm².
Significant differences (p<0.05) in coliforms were observed in Kampala central with the mean of 2.4 log10 CFU/ cm². However, no significant difference (p>0.05) in coliforms were observed for the hand samples in Nakawa and Kawempe divisions. Hand assessment of 12 samples (n=30) (40%) indicated no detection for S. aureus while 18 (60%) were above 2 log10 CFU/cm². Highest S. aureus count of 5.1 log10 CFU/cm² was observed in Kawempe while Nakawa hand samples showed no detection (Figure 3c). However, no significant difference (p<0.05) in S. aureus count was observed for Kawempe and Kampala central samples. Escherichia coli, Salmonella and Listeria spp were not detected on dispenser, refrigerator or hand samples.
3.3 Biochemical identification of suspect isolates
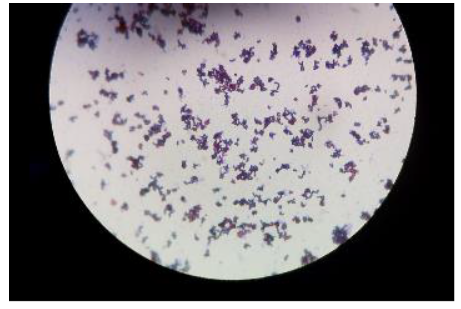
Findings from the IMVIC tests and sugars fermented are presented (Table 1). Acid production was indicated by the colour change of phenol red from red to yellow. The presence of gas bubbles in the Durham tube indicated the production of gas. No change in colour indicated a negative result. The isolates were Gram stained to find out whether they were Gram positive or negative (Figure 4).
+: positive; -: negative; A: acid; A+G: acid + gas
Table 1: Gram reaction and biochemical identification of isolates from fresh fruits, juice contact surfaces and hands.
3.4 Methicillin resistant Staphylococcus aureus (MRSA) from hand sample
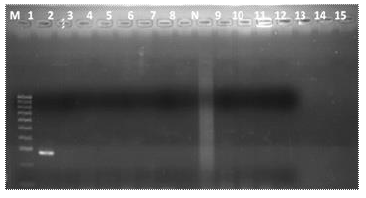
Only one sample out of 15 (6.7%) isolates was positive for the mecA gene. The coagulase positive hand isolate with the mecA gene was observed to amplify at 293bp (Figure 5).
4. Discussion
High aerobic counts on passion fruit, mango and pineapple samples could possibly be due to the high level of air and water pollution and human contact, which contaminated the fruits compared to the other areas. Fruit surfaces were possibly polluted by air which includes particulate dust, exhaust fumes, industrial fumes. The fruits could also be contaminated with dirty water because they were placed on the ground in the open markets so were liable to surface runoff often mixed with sewage and sludge. Samples had counts above 5 log10 CFU/cm² permitted for fresh fruit surfaces [18]. Microorganisms in the range of 10² to 109 colony-forming units (CFU) per gram exist on the surfaces of fresh fruits, grains and vegetables [19]. Microbial contamination with spoilage or pathogenic microorganisms on fruits like pineapples most likely comes from the ground to the rinds of these fruits [20].
Passion fruit samples were significantly different (p<0.05) in coliform counts from pineapples and mangoes possibly due to the use of the T-bar trellis system during growing and the use of nets in harvesting signifying good agricultural practice [21]. Non-significant differences (p>0.05) in coliform counts for mango and pineapple samples could possibly be due to obtaining the fruits from the same growing areas and export sources [21]. Escherichia coli on the fruit samples were possibly from the field or were contaminated with surface runoff mixed with sewage. Juices produced due to mechanical damage of the pineapple fruits could have attracted flies that contributed to contamination [22]. The presence of S. aureus on the fruit samples could possibly be due to human manipulation along the food chain. Passion fruit, mango and pineapple samples were not significantly different (p>0.05) in S. aureus counts in the three divisions. Lower S. aureus counts possibly imply good hand hygiene and use of good manufacturing practices for fruit samples in Kampala central.
The Government Regulation 962 of 2012, promulgated under the foodstuffs, cosmetics and disinfectants Act, No.54 of 1972 of the Republic of South Africa states an acceptable limit of 2 log10 CFU/cm² for APCs on food contact surfaces and processors’ hands [23]. An aerobic plate count ranging from 3.8 log10 CFU/cm² to 9.7 log10 CFU/ cm² was observed for 150 refrigerator surfaces for a study conducted in Khartoum North, Sudan [24]. In another study aerobic plate counts ranging from 0 to 14.1 CFU/mL for 180 household refrigerators have been reported in Nigeria [25]. The mean values of APCs obtained in Kampala central and Kawempe were higher than those obtained in other studies for processors’ hands. For instance, lower APC mean counts of 5.6 log10 CFU/cm², 5.5 log10 CFU/cm² and 5.3 log10 CFU/cm², were observed from processors’ hands from three areas in Benin city, Nigeria, however, they were also above the acceptable limit [10]. High bacterial counts of 3.9 log10 CFU/ cm² above the acceptable limits for hand samples have also been reported in South Africa [26]. High numbers of aerobic plate counts from juice contact and processors’ hands are possibly from air, dust, environment, fruits and equipment along the food chain. High APCS may probably indicate insufficient washing and sanitation of hands of personnel in these establishments.
The absence of aerobic mesophiles on some dispenser and refrigerator surfaces probably indicates proper sanitation, good hand hygiene of handlers and application of good manufacturing practices. Presence of coliforms on refrigerator surfaces may possibly be due to storage of several foods including poultry and meat whose leakages could possibly contaminate the fresh produce [24]. The presence of coliforms in 150 refrigerator surfaces with a range of 0.06 to 7.65 log10 CFU/ cm² has been reported [24]. Use of the same cleaning materials (dish clothes and sponges) for all surfaces, could also contribute to post processing contamination [27].
Coliforms observed in this study possibly indicate recent fecal contamination from hands used to clean the surfaces or they may come from common plant shoot, root, flower and fruit colonizers [28]. Lambrechts et al. states the complete absence of coliforms in samples obtained from hands or food contact surfaces. Coliforms may also indicate unsanitary conditions, unhygienic practices or use of poor quality water [29]. The use of contaminated hands resulting from cross-contamination in food products is likely to cause food borne diseases [30]. Cleaning and washing stage of sanitation were cited to remove one log order of total surface microbes [31]. Staphylococcus aureus was not detected in hand samples from Nakawa, which findings are similar to those obtained by [26].
Methicillin resistant strains were found in one hand sample from our study. This result indicates the possibility of the mecA gene within the community. Possible reasons for the presenc of this virulence gene could be due to contact with working surfaces, domestic animals, money or even during washing of utensils. Horinzontal transfer could also occur from the fruits or working surfaces during juice processing. Similar result in one out of 18 (5.6%) human swabbed samples was positive for the mecA gene [32]. Studies on the carriage of the MecA gene in S. aureus in food products have been done; for instance, 23 out of 41 (56.1%) isolates in bulk can and raw milk products were obtained [33]. Twenty percent isolates in raw milk [34] and one coagulase positive isolate out of 199 in fresh sea food were obtained [35].This can be a very serious source of disease or resistance to antibiotics for the population. However, since only a limited number of samples were done, much more investigations, still need to be done.
5. Conclusion
Based on our findings, pathogens such as MRSA are present on vendors’ hands and could be transmitted to fresh juices. Dwellers and travelers within the East African community need to be careful, where they purchase their products to minimize the likely occurrence of food borne illnesses. This is more so seen around the East African community when buses, trucks and aeroplanes leave the cities each day to transport passengers within the interconnected countries. Refreshments made from contaminated fruits, and kept in unhygienic contact surfaces may endanger the health of consumers during travel and within the community. Therefore, good handling and hygienic practices of fruits and juice contact surfaces as well as proper hand hygiene are crucial to the production of safe juice products as they are part of the food and beverage industry.
Acknowledgements
Technical assistance from the departments of Microbiology and Molecular Biology, College of Veterinary Medicine, Applied Resources and Biosecurity (COVAB), Makerere University, Kampala, Uganda is greatly appreciated. The work was funded by DAAD/RUFORUM (German Academic Exchange Service/ Regional Universities Forum for East and Southern Africa).
References
- Tambekar DH, Jaiswal VJ, Dhanorkar DV, et al. Microbial Quality and safety of street vended fruit juices: A case study of Amravati city. Internet J of Food Safety 10 (2009): 72-76.
- Semwanga, centre. Final report study for fruit sub-sector (Pineapples, Passion fruits, Mangoes) (2007): 343-353.
- Bello OO, Bello TK, Fashola MO, et al. Microbiological quality of some locally-produced fruit juices in Ogun State, South western Nigeria. J of Microbiol Research 2 (2014): 1-8.
- Asha S, Nithisha K, Niteesha G, et al. Evaluation of Microbial Quality of street vended vegetable and fruit juices. Int Research J of Biol Sci 3 (2014): 60-64.
- Abdalla MA, Suliman E, Alian YYH. A study of the microbial content of the domestic refrigerators in Khartoum area (Khartoum North). Sudan J of Vet Sci and Animal Husbandry 47 (2008): 15-23.
- Khan MM, Mehadi T, Chowdhury M, et al. Assessment of microbiological quality of some drinks sold in streets of Dhaka University Campus in Bangladesh. Int J of Food Cont 2 (2015): 1-4.
- Torres-Vitela MDRCA, Gómez Aldapa JF, Cerna-Cortes A, et al. Presence of indicator bacteria, diarrhoeagenic Escherichia coli pathotypes and Salmonella in fresh carrot juice from Mexican restaurants. Letters in Applied Microbiol 56 (2013): 180-185.
- Addisu D, Letebrhan K, Siyane S. Isolation and Identification of Bacteria from Fresh Fruit Juice Prepared in Cafeterias and Restaurants, Axum Town. Int J of Integrative Sci Innovation and Tech 5 (2016): 5-10.
- Imathiu SM. Street vended foods: Potential for improving food and nutrition security or a risk factor for foodborne diseases in developing countries? Current Research in Nutrition and Food Sci J 5 (2017): 55-65.
- Okareh OT, Erhahon OO. Microbiological Assessment of Food and Hand-Swabs Samples of School Food Vendors in Benin City, Nigeria. Food and Public Health 5 (2015): 23-28.
- International Organization for Standardization. International standard 18593. Switzerland (2004): 1-14.
- Morton D. Aerobic plate counts. In Eds.: Frances P Downes, Ito K. Compendium of Methods for the Microbiological Examination of Foods. Fourth Edition. American Public Health Association (2001): 63-66.
- Kornacki JL, Johnson JL. Enterobacteriaceae, Coliforms and E. coli as quality and safety indicators. In Eds.: Frances P Downes, and Ito K. Compendium of Methods for the Microbiological Examination of Foods. Fourth Edition. American Public Health Association (2001): 69-81.
- Lancette GA, Bennett RW. Staphylococcus aureus and Staphylococcal Enterotoxins. In Eds.: Frances P. Downes and Ito K. Compendium of Methods for the Microbiological Examination of Foods. Fourth Edition. American Public Health Association (2001): 387-400.
- Andrews WH, Russell S, Flower S, et al. Salmonella. In Eds.: Frances P Downes and Ito K. Compendium of Methods for the Microbiological Examination of Foods. (4th). American Public Health Association (2001): 357-377.
- Ryser D. Listeria. In Eds.: Frances P Downes, Ito K. Compendium of methods for the microbiological examination of foods. Fourth Edition. American Public Health Association (2001): 343-353
- Chikkala R, Nikhil OG, Kamaraju SR, et al. Heterogeneity in femA in the Indian isolates of S.aureus limits its usefulness as species marker. Advances in Infectious Diseases 2 (2012): 82-88.
- Al-Jedah JH, Robinson RK. Nutritional Value and Microbiological Safety of Fresh Fruit Juices sold through Retail Outlets in Qatar. Pakistan J of Nutrition 1 (2002): 79-81.
- Breidt F, Caldwell JM. Survival of Escherichia coli O157:H7 in cucumber fermentation brines. J of Food Sci 76 (2011): 198-203.
- Leite Caroline Junqueira, Barcellos Pereira de Sousa Jossana, Medeiros JA, et al. Inactivation of Escherichia coli, Listeria monocytogenes, and Salmonella Enteritidis by Cymbopogon citratus D.C. Stapf. Essential oil in Pineappple juice. J of Food Prot 79 (2016): 213-219.
- Sonko R, Njue E, Sebuliba J, et al. The horticultural sector in Uganda. (Ed.: Scipta Horticulturae) Number 1 (2005): 1-90.
- Babalola OO, Obashola E, Gawande RE. Microbiological quality control study of some processed fruit juices by conventional approach. Life Sci J 8 (2011): 18-24.
- Republic of South Africa. Regulation 962 of 2012: Regulations governing general hygiene requirements for food premises and the transport of food, promulgated under the Foodstuffs, cosmetics and Disinfectants Act No. 54 of 1972. Pretoria (2012).
- Oluwafemi F, Akpoguma S, Oladiran T, et al. Microbiological Quality of Household Refrigerators in Three Cities South. Microbial and Biochemical Tech 7 (2015): 206-209.
- Lambrechts AA, Human IS, Doughari JH, et al. Bacterial contamination of the hands of food handlers as indicator of hand washing efficacy in some convenient food industries. Pakistan J of Medical Sci 30 (2014): 755-758.
- Redmond EC, Griffith CJ. The importance of hygiene in the domestic kitchen: Implications for preparation and storage of food and infant formula. Perspectives in Public Health 129 (2009): 69-76.
- Barth M, Hankinson TR, Zhuang H, et al. Microbiological spoilage of fruitsand vegetables in Compendium of the microbiological spoilage of foods and beverages. Food Microbiol and Safety (2009): 125-182.
- Nma NO, Ola A. Microbiological analysis of some packaged fruit juices sold in port Harcourt Metropolis, Nigeria. Nature and Sci 11 (2013): 30-40.
- Lee HK, Halim HA, Thong KL, et al. Assessment of Food Safety Knowledge, Attitude, Self-Reported Practices and Microbiological Hand Hygiene of Food Handlers. Int J of Environ Research and Public Health 14 (2017): 1-14.
- Ali R, Hayat A, Fatima M, et al. Detection and enumeration of Enteric bacteria associated with food handlers and surfaces of food manufacturing industry located in Hub city, Pakistan. World Scientific News 49 (2016): 192-203.
- Vyetelova M, Vlkova H, Manga I. Occurrence and characteristics of Methicillin Resistant Staphylococcus and Methicillin Resistant coagulase negative Staphylococci in raw milk manufacturing.Czech J of Food Sci 29 (2011): 11-16.
- Asiimwe BB, Baldan R, Trovato A, et al. Prevalence and molecular characteristics of Staphylococcus aureus including methicillin resistant strains, isolated from bulk can milk and its raw milk products in pastoral communities of South West Uganda. BMC Infectiuos Diseases 17 (2017): 1-8.
- Riva A, Borgi E, Cirasola D, et al. Enterotoxin characterization and antimicrobial resistant patterns. J. of Food Prot 78 (2015): 1142-1146.
- Kumar L, Kasim A, Lekshmi M. Incidence of Staphylococcus aureus in fresh seafood. Advances in Microbiol 6 (2016): 399-406.
Journals List
© 2016-2024, Copyrights Fortune Journals. All Rights Reserved