Atypical Postnatal Hemorrhage in a Hemophilic Newborn: A Case Report and Parents’ Perspective Seven Years Later
Article Information
C Felice Civitillo1,2*, F Barcos-Munoz1, A Bordessoule2, O Karam3
1Neonatal Intensive Care Unit, Geneva University Hospital, Geneva, Switzerland
2Pediatric Intensive Care Unit, Geneva University Hospital, Geneva, Switzerland
3Section of Pediatric Critical Care Medicine, Department of Pediatrics, Yale School of Medicine, New Haven, CT, USA (ORCID 0000-0001-6606-1736)
*Corresponding author: C Felice Civitillo, Neonatal Intensive Care Unit, Geneva University Hospital, Geneva, Switzerland.
Received: 26 October 2022; Accepted: 02 November 2022; Published: 5 January 2023
Citation: C Felice Civitillo, F Barcos-Munoz, A Bordessoule, O Karam. Atypical Postnatal Hemorrhage in a Hemophilic Newborn: A Case Report and Parents’ Perspective Seven Years Later. Archives of Clinical and Biomedical Research 7 (2023): 01-07.
Share at FacebookKeywords
Postnatal Hemorrhage; Newborn
Article Details
1. Case Report
A.P. is a boy born to a 2G 0P 34-year-old mother, at 40 5/7 weeks of gestation. After two failed vacuum extractions, a caesarean section was performed due to engagement failure. Apgar score was 9/10/10 and umbilical cord pH of 7.21 and 7.29. Birth weight was 3960 g (P50-75), length 52 cm (P50-90), and head circumference (HC) 35 cm (P50-90). As for all emergent caesarean sections, a pediatric resident examined him immediately after birth.
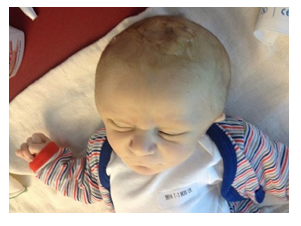
During the first hours of life, the parents report an irritable child who demonstrated signs of pain and discomfort. At 30 hours of life, the infant was found uncomfortable and pale, with a 4 cm increase in his HC, reason for which he was transferred to the Neonatal Intensive Care Unit (NICU). the newborn was extremely pale, irritable, and in apparent pain. The physical exam revealed a large, hard, pitting oedema over the skull, with a vacuum extraction mark (figure 1) and protruding ears.
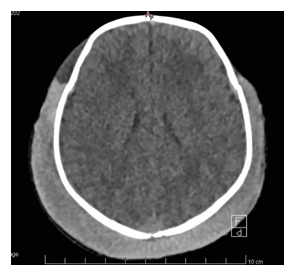
No fluctuating mass was identified. Pulse oximetry was 100% on room air, with mild respiratory distress and grunting, respiratory rate 45 per minute, heart rate 135 bpm and blood pressure was 66/32 mmHg (mean 43 mmHg). The admission blood gas showed a severe metabolic acidosis (pH 6.84, pCO2 4.6kPa, HCO3- 5.6 mmol/L, BE -22 mmol/L) with severe hyperlactatemia (23 mmol/l) and severe anemia (Hemoglobin (Hb) 46 g/l). Within minutes of NICU admission, the newborn became apneic and required bag-mask ventilation before emergent intubation. An umbilical venous catheter was emergently inserted, and he immediately received a 30 ml/kg of O negative red blood cells transfusion, as well as a 15 ml/kg plasma transfusion and 30 mg/kg fibrinogen concentrate. Calcium gluconate and sodium bicarbonate were also administered. The newborn was not actively warmed during the resuscitation and placed subsequently on therapeutic hypothermia (34°C) even if he didn’t meet the usual neonatal criteria for hypothermia. Blood coagulation tests were severely abnormal at admission, showing a prothrombin time (PT) 35%, an activated partial thromboplastin time (aPTT) >160 sec, and a fibrinogen level at 1.2 g/l. A head ultrasound (US), performed to rule out intracranial hemorrhage, showed diffuse echogenic modifications in the extracranial soft tissues, without a definitive diagnosis. An emergent head CT scan suggested a subgaleal hemorrhage (figure 2).
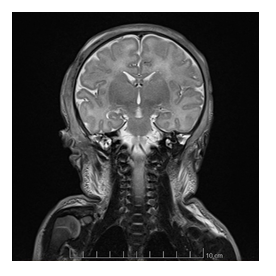
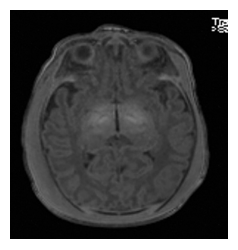
After the initial resuscitation, the newborn was actively cooled and his neurological status was grossly abnormal, with a Sarnat score fluctuating between 2 and 3, axial and peripheral hypotonia, absence of spontaneous movements, weak grasping, and no suction. Abnormal movements were also present, clinical seizures were suspected and treated with phenobarbital, but the EEG was impossible to interpret due to the thick oedema around the skull. After 72 hours, we discontinued hypothermia and gradually rewarmed the baby. The neurological status progressively normalized. The cerebral magnetic resonance imaging (MRI) performed at day 4 of life was normal; no ischemic lesions were identified (figures 3 and 4).
However, despite normothermia, coagulation tests remained abnormal, with a PTT increasing progressively to 127 seconds on the fifth day of life. Specific coagulation factors were then evaluated. Since factor VIII activity was less than 1%, severe hemophilia A was diagnosed. Family history was negative for hemophilia; however, a heterozygotic mutation of the F8 gene, responsible for the factor VIII production, was later found in the mother.
The atypical presentation of this extracranial massive hemorrhage and shock and the delayed diagnosis were caused by hemophilia. Hemophilia has without doubt contributed to the unusual presentation of this extracranial massive hemorrhage and hypovolemic shock, contributing to a delayed diagnosis.
2. Parental Experience
Seven years later, we contacted the family to understand their perception and comprehension of the initial events. The mother told us “Once they recognized my son wasn’t doing well, they took him to the NICU. As soon as I got there, I saw many doctors around him and even one running to him. That’s when I understood it was really serious. For a long time, no one came to talk to me. Then, doctors brought me to a small room and told me they believed a subgaleal hemorrhage was the origin of the hemorrhagic shock. They told me he was in an induced coma and was going to be cooled for three days. They also told me he would likely suffer from cerebral injury which would have long-term consequences, the magnitude of which were unknown. At that moment, I had the impression I was in a nightmare and I was hoping I would soon wake up. Time stopped, we were in a suspended reality, nothing counted but the updates we received.” When asked about the way we communicated, the mother responded “It seems doctors still aren’t taught how to deal with these situations, what to say and how to say it. Some were more direct and clearer than others. I would like to focus on those who had the best communication skills. They told us things as they were, including their own uncertainties and doubts. They were also not afraid to talk openly about medical errors.” The mother also provided feedback on how things could be improved: “I believe some things could have been done differently. My son bled from 30 hours before it was recognized. There were at least four different shifts that could have evaluated his discomfort and perhaps prevented this outcome. This is the part that will never heal. I don’t want another child to have to go through this. Here are some aspects I think should be addressed. It is important to create a better connection between the child’s electronic medical record and his mother’s. The parents’ input should be sought instead of discarded. The babies should be examined more thoroughly, and any issue should be escalated instead of minimized. Things might also have been better if there were more nurses and midwifes. I think it is also important to realize that there isn’t a one-size-fits-all approach and that guidelines should be tailored to the situation. In the newborn nursery and even in the NICU, it is important not to separate the babies from their parents. Their care must be co-managed. I also believe that the hematologist (in our case) should have been consulted earlier, as soon as something doesn’t seem right. And finally, when a parent asks what has been put in place to avoid this reoccurring, please do not be disrespectful and suggest seeing a psychologist.” The parents also told us how they were doing now: “Seven years after these events, my son is doing well. He is an intelligent, affectionate, and very happy young boy. The disclosure of his hemophilia was felt as a huge relief. Everything made sense. Compared to other parents, we embraced the diagnosis. Our son was alive, and by then, we already knew his head CT was normal. We could deal with everything else. I don’t know how he was affected by this, but when I first told him about his neonatal history, he spontaneously put his hand to the exact spot where his subgaleal hemorrhage was the worst. I was shocked his body seemed to remember. Personally, this whole situation affected my trust in the healthcare system. It also complicated the post-natal bond with my son, but that resolved over time.”
3. Subgaleal Hemorrhage
3.1 Epidemiology
The suspected subgaleal hemorrhage (SGH) is a potential life-threatening event that is often under-recognized and under-diagnosed [1]. It is the consequence of the rupture of emissary veins between the scalp and dural sinuses, often caused by scalp traction during delivery [2,3]. Its general incidence is 0.2-3 per 1000 live births [4,5], and 6 to 41 per 1000 live births after instrumental delivery [6]. The subgaleal space extends with no anatomical limits across the cranial vault from the frontalis muscle and the orbital ridges to the posterior nuchal lines and laterally, at the level of the ears, to the temporalis muscle. Due to lack of anatomical limits, a lesion in this region can cause a blood loss from 50 to up to 260 ml in the subgaleal space [4]. This might represent 20-40 percent of a term neonate's blood volume, with a considerable risk of hemorrhagic shock and death [3,4]. The reported mortality varies from 12 to 25% [5-8]. Instrumented delivery, in particular vacuum extraction (VE) over forceps, represents the main risk factor for SGH (OR = 7.17) [9], although SGH can occur after spontaneous delivery. The use of vacuum extraction has increased over the past three decades and this has also caused an increased incidence of SGH in the developed world [2]. Up to 5–10% of all deliveries are estimated to be performed by vacuum extraction [11].Elements that may increase the risk of SGH are the type and size of the vacuum cup and its location and duration of application, the level of negative pressure, and the direction of traction, as well as the operator experience [6].The risk of SGH are further increased after failed vacuum extraction [6].
3.2 Diagnosis and Management
Early recognition and aggressive treatment of SGH are crucial as worse outcome were reported with delayed diagnosis [6]. Referral to a hospital with a neonatal intensive care may be necessary, but this might worsen the prognosis, as the transfer itself is perilous [6]. Diagnosis is mostly clinical, especially since the differential diagnosis between caput succedaneum, cephalohematoma and SGH might be challenging for those with less experience. The usual onset of symptoms is within the first hour after birth, but some have reported up to 4 days of life in less severe cases [8]. For these reasons, a close surveillance is recommended after instrumental delivery, based on clinical surveillance, head circumference (although it can fluctuate after birth), and vital signs. In doubt, admission to the NICU for close surveillance seems appropriate [2]. Chang et al. found in their series that patients with early increase in head circumference had a better outcome, as the diagnosis was made earlier, usually because of abnormal vital signs [6]. If in doubt, hemoglobin (Hb) level should be measured as soon as possible [13], keeping in mind that it takes time for Hb to drop, as the initial bleeding event caused a loss of both Hb and volume. Therefore, lower Hb is the result of hemodilution, which takes time (extravascular to intravascular shift, decreased urine output) [8,13]. Head circumference (although it can fluctuate after birth), blood pressure and heart rate should be closely monitored as initial signs of shock may be insidious and treatment should not be delayed [12,13]. Therapy includes management of hypovolemic shock with volume, with red blood cell and plasma transfusions to correct hypovolemia, anemia, and coagulation abnormalities [8].
3.3 Outcomes
The risk of adverse outcome is well known in a setting of SGH: the mortality rate reported in the until the nineties was up to 17-25% [5,7], while it is has lowered to 5-14% [4,6] in more recent studies, mainly thanks to close monitoring and early diagnosis.
4. Hemophilia
Coagulation disorders in the neonatal period can explain atypical and more severe manifestations of traumatic birth complications. Acquired disorders, typically seen in sick neonates, because most hemorrhagic manifestations observed in the newborn, but inherited coagulation disorders, such as hemophilia, can also have a neonatal presentation. Hemophilia is an inherited X-linked coagulation disorder, whose gene is located at the tip of the long arm of chromosome X [14]. It can be classified in Hemophilia A or B, according to the deficiency on factor VIII or IX, respectively. It is the most frequent hereditary coagulation disorder to present during the neonatal period. Approximately two-thirds of the patients have a positive family history, the others being due to sporadic mutations in the gene for factor VIII. Its severity can be classified according to the plasma factor activity in: severe (<1%), moderate (1 to 5%), or mild (>5 to 40%). The most common type is hemophilia A that accounts for about 85% of the cases, with an incidence of 1 per 5,000 male births; hemophilia B is less frequent (1 per 20,000 males) [14]. In 1966, only 10% were diagnosed in the neonatal period [16]; in the nineties, the proportion of neonatal diagnoses was between 38% and 54% [17]. Fifty years later, in 2016, Jaffray et al. reported that almost 70% of children were diagnosed in the first month of life [19]. An unexpected bleeding event associated to an isolated prolonged activated partial thromboplastin time (aPTT) in a male infant [20] can raise a clinical suspicion of hemophilia. A decreased level of factor VIII (hemophilia A) or IX (hemophilia B) activity, the other coagulation factors, including von Willebrand factor, being normal can confirm the diagnosis. Laboratory diagnosis of hemophilia is possible at birth, even in case of hemophilia-carrier mothers, as factors VIII and IX do not cross the placenta. All male newborns of known hemophilia-carrier mothers should undergo clotting factor assays in cord blood (from a vein on the fetal side of the placenta). This test can be performed on peripheral blood when cord blood is not available [21]. The factor’s activity level defines the severity of the disease and correlates with clinical outcomes. The severity of hemophilia A can be diagnosed at birth, as factor VIII does not cross the placenta and levels at birth in term and preterm infants are similar to adult values (50-150 IU/dl). On the other hand, hemophilia B during the neonatal period, particularly mild forms, may be difficult to exclude in newborns. In a setting of a positive familiar history, prenatal diagnosis is possible.21 Fetal sexing, performed by maternal blood testing around 10 weeks of gestation or by fetal ultrasound between 18 and 20 weeks, can guide management of pregnancy and delivery. Chorionic villus sampling, between 11 and 13 weeks of gestation, is the most widely used method to determine if a male fetus is affected from hemophilia [21]. In the second trimester, two diagnostic methods are available: around 16 weeks, DNA analysis on amniotic fluid and around 18-20 weeks cordocentesis may allow analysis of fetal blood (factor VIII:C and F IX:C) [21,22].Finally, to avoid the risk of first trimester miscarriage due to prenatal diagnosis, third trimester amniocentesis is an option, carrying about 1% risk of early delivery [22]. Pattern of bleeding in newborns is different than in older children with hemophilia, where muscle and joints bleeding are the typical manifestations (as soon as they move, stand, and walk). During the neonatal period, the main sources of bleeding are ‘iatrogenic’: e.g., venopuncture, intramuscular vitamin K administration or heel sticks [15].Umbilical stump bleeding, a frequent manifestation in fibrinogen and factor XIII deficiencies, is rare in hemophilia. Gastrointestinal bleeding is rare but can be serious (<5% of cases) [15,21]. Intracranial hemorrhage (ICH) and extracranial hemorrhage (ECH), including SGH and cephalohematoma, are the most severe hemophilia complications in newborns. ECH is usually related to birth trauma and may occur irrespective of the mode of delivery. Although ICH has been reported in 1-4% of hemophilic newborns [16], the precise incidence is unknown. Kulkarni and Lusher, in their review performed in 1999 [20], describe the clinical presentation of ICH and ECH as often non-specific and subtle, similar to neonatal meningitis, sepsis or disseminated intravascular coagulation. In both ICH and ECH, common signs included anemia, lethargy, hypotension, and shock. Signs of ICH can be more specific with bulging fontanel, seizures, and alteration of the neurological status. The reported cumulative incidence of ICH and SGH in newborns with hemophilia A and B was 3.6%, and the mean age of diagnosis was 4.5 days [20]. Recent studies are consistent with these data and show a cumulative incidence of ICH and ECH of 3.5-5% [23] ICH and in particular subdural hemorrhage are reported more commonly than ECH [21]. Richards et al evaluated the risk of symptomatic intracranial hemorrhage 44 times higher in newborns with hemophilia [23].In Klinge et al, a series of 27 patients with hemophilia and ICH, bleeding was diagnosed at birth in 11 cases [24]. The precise anatomical location of the ICH remains often unspecified in publications [21].In rare cases, neonatal hemophilia presents with subgaleal hemorrhage that carries a high risk of severe bleeding and death [24]. An optimal perinatal management of suspected or confirmed cases should reduce the risk of both neonatal and maternal bleeding and should imply a delivery in a perinatal center with hematologic expertise [22].Instrumental delivery, and in particular vacuum extraction, should be avoided as it increases the risk of injuries and bleeding compared to spontaneous vaginal delivery. Elective cesarean section is still controversial, as some studies report improved outcomes [26], whereas others do not. In one study, performed by Michaud on 87 patients, the overall hemorrhagic risk was 10% for vaginal delivery, 64% for vacuum extraction and 23% for cesarean section [26]. Due to lack of data, management of breech presentation at term in infants with hemophilia remains unclear [22].Therefore, the ideal mode of delivery remains controversial [27,28]. However, some precautions might seem reasonable: vacuum extraction, rotational and mid cavity forceps should be avoided in case of antenatal diagnosis of hemophilia [22],blood samples should not be taken from the fetal scalp, and any other action that could lead to bleeding, including intramuscular injection of vitamin K, should be avoided [15].
5. Parental Feedback
Communication between parents and physicians in the NICU is critical as it takes place in a highly stressful environment and deals with severe illness and death. Wool et al. [29] describe in their review that most parents want to be the final decision-maker for their critically ill child; this process of parental decision making is mainly driven by family-clinicians communication, but the support systems, parental emotions, and the child's clinical status influence significantly this decision-making process. In addition, several authors describe that there is a high rate of acute stress and posttraumatic stress symptoms among children and parents after admission to NICU [30-32]: these data are consistent with Nelson’s [33] findings for parents of older children, admitted in a pediatric intensive care unit (PICU). Rissman [34] et al. show that most parents and physicians report having had discussions about prognosis, primarily length of stay and physical morbidity after ICU. The concordance of this discussion between parents and physicians is suboptimal. All the above can be explained by a descriptive study by Jones [35] et al. who have found obvious qualitative differences between the content discussed by residents compared to other types of doctors. The most experienced health personnel (nurses, trainees and attending physicians) discussed prognosis and worst-case scenarios and they were more responsive to family emotions, while residents avoided discussing prognosis and the possible unfavorable outcomes with families and felt less prepared to face the emotional reactions of parents to an uncertain diagnosis. Moreover, they were often interrupted during parental meetings. This family’s experience underlines how useful can be a clear and appropriate communication while coping with a medical error: it can also help to reestablish trust and therapeutic alliance, as Spanakis shows in his work [36]. At the time, parents were not allowed to be present during their child’s resuscitation. These parents were not allowed to assist to their child’s resuscitation. Seven years later, our unit authorizes, and even encourages, caregivers’ presence during resuscitation and critical situations. Based on their testimony, observing their child’s resuscitation might have benefited this family.
6. Conclusion
The presence of a subgaleal hematoma, in itself, has a high mortality and if we add a congenital clotting disorder such as hemophilia A, the prognosis could be even worse. A prompt diagnosis and a high degree of suspicion (if risk factors are present) are needed. In conclusion, hemophilia A explains, retrospectively, the subacute but massive extracranial bleeding in our patient. Most likely, small vessel injuries continued to bleed slowly due to inappropriate clotting, leading to the progressive hypovolemic shock. Hemophilia should always be suspected in a setting of hemorrhage with persistent prolonged aPTT. Relationship with parents should be open and sincere, even in case of uncertain diagnosis or prognosis, without paternalistic nor condescending attitudes.
Acknowledgments
We are grateful to our patient’s parents who have been tremendous partners over the years. The patient’s parents authorized the publication of this case and asked us specifically to use photos without hiding their son’s eyes
References
- Uchil D, Arulkumaran S. Neonatal subgaleal hemorrhage and its relationship to delivery by vacuum extraction Obstet Gynecol Surv 58 (2003): 687-693.
- Colditz MJ, Lai MM, Cartwright DW, et al. Subgaleal haemorrhage in the newborn: a call for early diagnosis and aggressive management. J Paediatr Child Health 51 (2015): 140-146.
- Hangai M1, Kimura Y, Mori H, et al. Computed tomography findings of ongoing subgaleal hemorrhage Pediatr Int 56 (2014): 623-626.
- Swanson AE, Veldman A, Wallace EM, et al. Subgaleal hemorrhage: Risk factors and outcomes. Acta Obstet. Gynecol. Scand 91 (2012): 260-263.
- Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal haematoma: associated risk factors, complications and outcome. J Paediatr Child Health 32 (1996): 228-232.
- Chang HY, Peng CC, Kao HA, et al. Neonatal subgaleal hemorrhage: clinical presentation, treatment, and predictors of poor prognosis. Pediatr Int 49 (2007): 903-907.
- Plauche W. Subgaleal hematoma. A complication of instrumental delivery. J Am Med Assoc 244 (1980): 1597-1598.
- Amar AP, Aryan HE, Meltzer HS, et al. Neonatal subgaleal hematoma causing brain compression: Report of two cases and review of the literature. Neurosurgery 52 (2003): 1470-1474.
- Colditz MJ, Lai MM, Cartwright DW, et al. Subgaleal haemorrhage in the newborn: A call for early diagnosis and aggressive management. J Paediatr Child Health 51 (2015): 140-146.
- Ali UA, Norwitz ER. Vacuum-assisted vaginal delivery. Rev. Obstet. Gynecol 2 (2009): 5-17.
- Davis DJ. Neonatal subgaleal hemorrhage: Diagnosis and management. CMAJ 164 (2001): 1452-1453.
- Ng PC, Siu YK, Lewindon PJ. Subaponeurotic haemorrhage in the1990s: A 3-year surveillance. Acta Paediatr 84 (1995): 1065-1069.
- Schierholz E, Walker SR. Responding to traumatic birth: subgaleal hemorrhage, assessment, and management during transport. Adv Neonat Care 10 (2010): 311-315.
- Orkin SH, Nathan DG, Ginsberg D, et al. eds. Nathan and Oski’s hematology of infancy and childhood. 7 ed. Philadelphia: WB Saunders (2009).
- Chalmers EA. Neonatal coagulation problems. Arch Dis Child Fetal Neonatal Ed 89 (2004): 475-478.
- Baehner RL, Strauss HS. Hemophilia in the first year of life. New England Journal of Medicine 275 (1966): 524±528.
- Conway JH, Hilgartner MW. Initial presentations of pediatric hemophiliacs. Archives of Pediatrics and Adolescent Medicine 148 (1994): 589±594.
- Pollmann H, Richter H, Ringkamp H. et al. When are children diagnosed as having severe haemophilia and when do they start to bleed? A 10-year single-centre PUP study. European Journal of Pediatrics 158 (1999): S166±S170.
- Jaffray J, Young G, Ko RH. The bleeding newborn: A review of presentation, diagnosis, and management Seminars in Fetal & Neonatal Medicine 21 (2016): 44-49.
- Kulkarni R, Lusher JM. Intracranial and extracranial hemorrhages in newborns with hemophilia: a review of the literature. Journal of Pediatric Hematology/Oncology 21 (1999): 289-295.
- Kulkarni R, Lusher J. Perinatal management of neonates with haemophilia. Br J Haematol 112 (2001): 264-274.
- Chalmers E, Williams M, Brennand J, et al. Guideline on the management of haemophilia in the fetus and neonate. Br J Haematol 154 (2011): 208-215.
- Richards M, Lavigne Lissalde G, Combescure C, et al. Neonatal bleeding in haemophilia: a European cohort study. Br J Haematol 156 (2012): 374e82.
- Klinge J, Auberger K, Auerswald G, et al. Prevalence and outcome of intracranial haemorrhage in haemophiliacs: a survey of the paediatric group of the German Society of Thrombosis and Haemostasis (GTH). European Journal of Pediatrics 158 (1999): S162± S165.
- MacLean PE, Fijnvandraat K, Beijlevelt M, et al. The impact of unaware carrier ship on the clinical presentation of haemophilia. Haemophilia 10 (2004): 560-564.
- Michaud JL, Rivard GE, Chessex P. Intracranial hemorrhage in a newborn with hemophilia following elective cesarean section. American Journal of Pediatric Hematology/Oncology 13 (1991): 473-475.
- James AH, Hoots K. The optimal mode of delivery for the haemophilia carrier expecting an affected infant is caesarean delivery. Haemophilia16 (2010): 420-424.
- Ljung R. The optimal mode of delivery for the haemophilia carrier expecting an affected infant is vaginal delivery. Haemophilia 16 (2010): 415-419.
- Wool J, Irving SY, Meghani SH, et al. Parental Decision-Making in the Pediatric Intensive Care Unit: An Integrative Review. Journal of Family Nursing 27 (2021): 154-167.
- Barnes S, Broom M, Jordan Z. Incidence and prevalence of acute stress disorder and post-traumatic stress disorder in parents of children hospitalized in intensive care units: a systematic review protocol. JBI Evid Synth 19 (2021): 236-241.
- Aftyka A, Rybojad B, Rozalska-Walaszek I, et al. Post-traumatic stress disorder in parents of children hospitalized in the neonatal intensive care unit (NICU): medical and demographic risk factors. Psychiatr Danub 26 (2014): 347-352.
- Aftyka A, Rybojad B, Rosa W, et al. Risk factors for the development of post-traumatic stress disorder and coping strategies in mothers and fathers following infant hospitalisation in the neonatal intensive care unit. J Clin Nurs 26 (2017): 4436-4445.
- Nelson LP, Lachman SE, Li SW, et al. The Effects of Family Functioning on the Development of Posttraumatic Stress in Children and Their Parents Following Admission to the PICU. Pediatr Crit Care Med 20 (2019): e208-e215.
- Rissman L, Derrington S, Rychlik K, et al. Parent and Physician Report of Discussions About Prognosis for Critically Ill Children. Pediatr Crit Care Med 22 (2021): 785-794.
- Jones AH, Jacobs MB, October TW. Communication Skills and Practices Vary by Clinician Type. Hosp Pediatr April 10 (2020): 325-330.
- Spanakis, Spiro G. Disclosure after medical error. Current Opinion in Anaesthesiology 34 (2021): 173-175.