Are Chemokines IL-8 (CXCL8) and MCP-1 (Monocyte Chemoatractant 1) Cooperating to Enhance Inflammation in Children with Sickle Cell Disease Living in Sub-Saharan area?
Article Information
Liliane K. Siransy1-2,*, Dasse S. Romualde1, Honoré Adou1, Sekongo Yassongui2, Patricia Kouacou1, Sidonie Kouamenan2, Richard Yeboah1, Saydou Kaboré2
1Félix Houphouet Boigny University- Medical Sciences -Immunology –Allergology Department, 1, boulevard de l’Université, Cocody, BP V 34 Abidjan, Ivory Coast
2National Blood Transfusion Center, Therapeutic and Research unit, 52, boulevard de Marseille, Zone 3, BP V 15 Abidjan, Ivory Coast
*Corresponding Author: Liliane Kouabla Siransy, Félix Houphouet Boigny University- Medical Sciences -Immunology –Allergology Department, 1, boulevard de l’Université, Cocody, BP V 34 Abidjan, Ivory Coast
Received: 15 May 2020; Accepted: 27 May 2020; Published: 12 June 2020
Citation: Liliane K. Siransy, Dasse S. Romualde, Honoré Adou, Sekongo Yassongui, Patricia Kouacou, Sidonie Kouamenan, Richard Yeboah, Saydou Kaboré. Are Chemokines IL-8 (CXCL8) and MCP-1 (Monocyte Chemoatractant 1) Cooperating to Enhance Inflammation in Children with Sickle Cell Disease Living in Sub-Saharan area?. Archives of Microbiology & Immunology 4 (2020): 66-74.
Share at FacebookAbstract
1. Introduction
ickle cell disease is a genetic disease very prevalent in the world and particularly in Sub-Saharan African countries. The prevalence ranges from 20% and 30%. In some African countries, it can reach 45% [1]. The number of newborns affected by this genetic disease is estimated at 240,000 per year or 0.3 to 25 per 1000 live births with fifty to seventy five percentages who do not reach the age of five [2–4]. In Côte d’Ivoire, country located in west Africa, the SCD prevalence rate is 12% [5]. As such, SCD is one of the greatest public health treat of all time and represent a public health problem. This disease makes patients more vulnerable to infections with high mortality, organs damage, and chronic anemia among children [6]. A better comprehension of the disease physiopathology in general will help to develop therapeutic approach to improve the management of the patients. There is an ongoing regular inflammatory status due to high levels of cytokines [7]. In this course, chemokines CXL and CCL also have been identified participating in this inflammatory process, particularly Interleukin-8 (IL8 or CXCL-8) and Monocyte Chemoattractant Protein-1, (MCP-1or CCL2). These two chemokines have been identified as having a chemotactic activity for neutrophils, monocytes, macrophages and lymphocytes [8]. Detection of inflammatory cytokines in the during non-crisis periods could be a guide for medical to improve management of SCD patients. This study strives to observe whether chemokines IL-8 and MCP-1 can be used to predict morbidity or mortality in SCD children.
Keywords
Sickle cell disease
Article Details
2.Patients And Methods
2.1 Study populationThis is a prospective case control study with 42 SCD patients from 4 to 18 years old followed at the Unite Thérapeutique transfusionnelle located in the Centre National de Transfusion sanguine in Abidjan, Côte d’Ivoire. It was conducted from October 2016 to February 2017 after approval from the national ethics board. Informed consents were obtained from the patients’ parents before the patient was enrolled in this study.
Documentation of homozygous or heterogynous sickle cell patients had been determined by hemoglobin electrophoresis on cellulose acetate strips (PH 9.2).
Patients were divided into two groups: patients in steady state for the first group and patients admitted for crisis for the second group. The steady state was defined by as a condition without crisis, illness or infection during the last three months and this group represents our control versus crisis group. Vasoocclusive crisis (VOC) was defined as an episode of diffuse acute pain almost in leg, arms, chest and abdomen with infection or anemia, necessitating hospital admission and or painkiller infusion.
Clinical investigations were assessed by hematology specialist. Plasma chemokines levels were compared in steady state patients and VOC patients. according to sex, gender, hemoglobin profile, hematological parameters and body mass index. All subjects were living in Côte d’Ivoire but belonging to different countries from West Africa (Nigeria, Togo, Ghana, Mali, and Guinea) but most from Côte d’Ivoire.
2.2 Chemokines assays
Blood sample were collected by veinopuncture in EDTA for the determination of the blood cell count and for the cytokine assay.
Blood count was obtained with the Sysmex XN 550 Hematology analyzer and then the plasma was separated from the tube sample at 1000g at 4°C for 10 min and stored at -30°c for chemokines assays.
Prior to use, the samples were thawed completely, mixed and centrifuged.
Plasma samples were measured by using BioLegend’s LEGENDplexTM Human Inflammation Panel assays which is bead-based immunoassays, using fluorescence encoded beads. This panel allows simultaneous quantification of many human inflammatory cytokines and chemokines, but in this study we focused on chemokines MCP-1 (CCL2) and IL-8 (CXCL8). The assay was performing using a filter plate in BioLegend’s laboratory in San Diego, US. All samples were analyzed on the same day after being thawed.
All samples were run and analyzed on the same day after being thawed completely. A minimum of 3000 positive beads for these chemokines was acquired with a cytometer type BD FACSCalibur™. Manufactured supplied controls were used. The assay sensitivity for IL-8 (CXCL8) and MCP-1 (CCL2) were respectively 1pg/mL and 1.1pg/mL, the intra assay precision and interprecision ranged from 5-9% for IL-8 and MCP-1. Data analysis was done using LEGENDplexTM Data Analysis Software when data acquisition is completed. The measurement ranges for MCP-1 and IL-8 was 0 to 10 000 pg/mL.
2.3 Statistical analysis
All results are expressed as mean +/- SD. Data were analyzed using SPSS version 22.0 program (SPSS Inc., Chicago, Illinois, USA). Statistical results with a P value ≤0.05 were significant. We used the Levene Test on equal variances. For the comparison of means, we used Student test when there was an equality of variances and either Man Whitney U test or Kruskal-Wallis when there was no equality of the variances.
3. Results
3.1 General characteristics of study subjects and circulating levels of chemokinesDemographics characteristics of the patients are summarized in Table 1. Data are reported as means (minimum and maximum) ± SD.
Table 1: Demographics characteristics
|
Parameters |
n |
Mean (min-max)± SD |
|
WBC (*109/l) |
33 |
12663, 63 (4800-22000) ± 4332, 06 |
|
Hb (g/dl) |
33 |
7, 59 (5, 5-9, 9) ± 1, 16 |
|
Neutrophils |
29 |
5501, 21 (1427, 40-11346) ± 2599, 46 |
|
Monocytes |
29 |
1119, 58 (421, 6-3496) ±616, 30 |
|
Hemoglobin type |
42 |
Percentages |
|
AFA2 |
8 |
19, 05% |
|
SAFA2 |
1 |
2, 38% |
|
SC |
1 |
2, 38% |
|
SFA2 |
11 |
26, 19% |
|
SSFA2 |
23 |
54, 76% |
42 SCD patients (23 HbSS homozygous type, 11 Sβ+ Thal heterozygous type, and 8 βThal homozygous type) from 4-18 years, attending the Unité de Thérapeutique transfusionnelle located within the Centre National de Transfusion Sanguine in Abidjan, were enrolled in this study.
22 SCD females (52, 38%) and 20 SCD males (47.62%) were evaluated in this study. 22 (52.38%) were in crisis, while 20 (47, 62%) were in a steady state condition. This last group represented our controls. The “healthy” group was constituted of 20 controls with 7 males and 13 females and mean age 12 years +/-3.8. The crisis group was constituted of 22 patients with 9 females and 13 males and the mean age 9 years +/- 4.08 (Table 2).
Table 2: IL-8 and MCP-1 levels in age and sex matched control
|
Items |
Crisis SCD patients (n=22) |
Steady state SCD patients (n=20) |
||
|
Age (years) |
Sex |
Age (years) |
Sex |
|
|
mean = 9 |
1, 44* |
mean = 12 |
0, 54* |
|
|
SD = 4, 81 |
SD = 3, 08 |
|||
|
IL-8 |
Spearman =-0, 199 |
p=0, 61 |
Spearman = 0, 555 |
p=0, 31 |
|
MCP-1 |
Spearman =-0, 095 |
p=0, 62 |
Spearman = 0, 606 |
p=0, 43 |
7 SCD patients with crisis (31.81%) have IL-8 levels under 10pg/mL and among them 3 have undetectable levels.
IL-8 was higher in steady state group subjects compared to crisis subjects (1946 pg/mL+1384 versus 403.31 pg/mL+827.67, p=0.001). For MCP-1, no statistical differences have been identified. (256.33 pg/mL+331.11 versus 261.72 pg/mL+324.27, p=0.9) (Table 3).
According to the hemoglobin type, circulating levels of IL-8 and MCP-1 are higher in AFA2 patients followed by SSFA2 and SFA2 patients (Table 4).
Table 3: Comparison of circulating levels of IL-8 and MCP-1 in SCD patients
|
Items |
n |
IL-8 (pg/ml) mean (min-max)± SD |
P value |
MCP-1 (pg/ml) Mean (min-max) ± SD |
P Value |
|
Age |
|||||
|
< 10 |
16 |
433, 86 (0-5475, 32) ±787, 32 |
0, 015* |
305, 88 (38, 57-1015, 13) ±375, 33 |
0, 46** |
|
≥10 |
26 |
1571, 59 (0-5475, 32) ±1464, 84 |
229, 99 (33, 86-1515, 52) ±291, 92 |
||
|
Sex |
|||||
|
F |
22 |
1474, 08 (3, 35-5475, 32) ±1461, 50 |
0, 09** |
350, 73 (33, 86-1515, 52) ±414, 00 |
0, 16* |
|
M |
20 |
768, 67 (0-475, 32) ±1162, 50 |
157, 89 (3, 35-5475, 32) ±128, 07 |
||
|
BMI |
|||||
|
OW |
8 |
964, 31 (0-2567, 45) ±1097, 64 |
0, 79**** |
437, 31 (59, 49-1015, 52) ±438, 15 |
0, 25*** |
|
NW |
16 |
1278, 43 (0-5475, 32) ±1627, 99 |
265, 93 (33, 86-1515, 52) ±371, 17 |
||
|
UW |
18 |
1098, 20 (0-3638, 6) ±1268, 74 |
157, 83 (42, 67-436, 54) ±121, 80 |
||
|
Clinical status |
|||||
|
Crisis |
22 |
403, 31 (0-3786, 98) ±827, 67 |
0, 001* |
256, 33 (33, 86-1015, 13) ±331, 11 |
0, 95** |
|
Steady state |
20 |
1946, 51 (5, 37-5475, 32) ±1384, 67 |
261, 72 (38, 57-1515, 52) ±324, 27 |
||
|
Hemoglobin type |
|||||
|
SSFA2 |
23 |
1161, 16 (0-2567, 45) ±1077, 98 |
0, 06*** |
283, 41 (33, 86-1015, 13) ±316, 41 |
0, 47 |
|
SFA2 |
11 |
428, 69 (0-3499, 83) ±1049, 08 |
158, 07 (38, 57-537, 95) ±142, 90 |
||
|
AFA2 |
8 |
2047, 60 (0-5475, 32) ±1964, 94 |
327, 06 (58, 71-1515, 52) ±497, 28 |
||
|
*Mann-Withney U test-** Test de student-*** Kruskal-Wallis-Test Anova**** |
|||||
Table 4: Means levels of IL-8, MCP-1 and BMI according to the hemoglobin type
|
SSFA2 |
SFA2 |
AFA2 |
|||||||
|
crisis (n=12) |
steady state (n=11) |
p |
crisis (n=7) |
steady state (n=4) |
p |
crisis (n=2) |
steady state (n=6) |
p |
|
|
IL-8 |
403.27 (0-2489.75) ±688.64 |
1987.95 (0-2567.45) ±770.46 |
0, 002 |
163, 66 (0-840.73) ±313, 15 |
892, 49 (5, 3-3499.83) ±1738, 32 |
0, 44 |
1819, 3 (0-3638) ±2572, 87 |
2123, 71 (130, 10-5475.32) ±2013, 36 |
0, 73 |
|
MCP-1 |
323.81 (33, 86-147, 79) ±424.09 |
239.34 (59.49-436.54) ±135.36 |
0, 28 |
197, 19 (51, 8-537, 95) ±168, 87 |
89, 62 (38, 57-113, 11) ±34, 85 |
0, 13 |
242, 17 (71, 21-413, 14) ±241,78 |
355, 36 (58, 71-1515, 32) ±575, 04 |
0, 73 |
|
BMI |
17.73 (11, 83-23.66) ±4, 4 |
17.29 (13.71-23, 53) ±4, 19 |
0, 88 |
17, 96 (13, 14-21, 89) ±2, 76 |
16, 79 (10, 32-21, 87) ±4, 95 |
0, 8 |
12, 81 |
16, 75 (12, 39-19, 89) ±3.32 |
0, 3 |
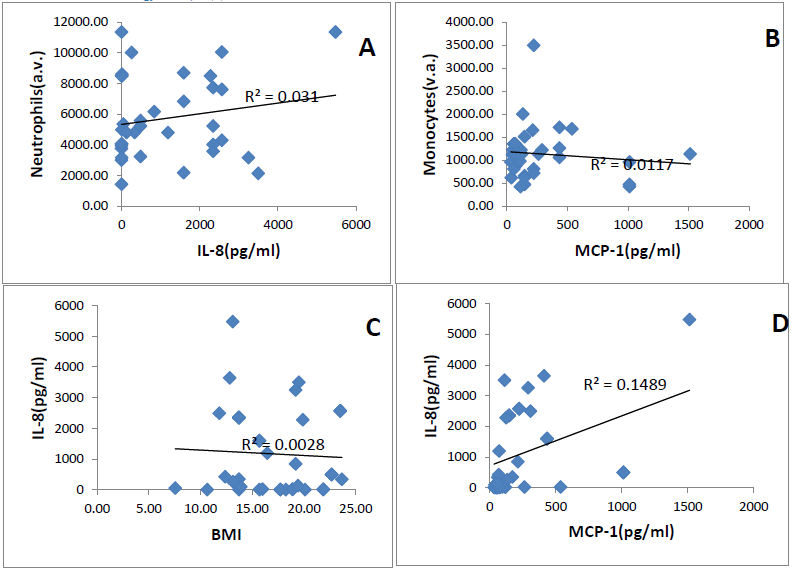
IL-8 and MCP-1 plasma levels were not correlated respectively with neutrophils count and monocytes count (r2=0.031, r2=0.001), (Figures 1A, 1B). Relationship between the BMI (Body mass Index) and plasma levels was determined by a regression analysis. Figure 1 C shows no correlation of the BMI and the levels of chemokines. (r2=0.028 for IL-8 and 0.02 for MCP-1). On the other hand, a positive slight trend is obtained between the two chemokines in both patients group (r2=0.14). A positive correlation was found for both chemokines according to age in the steady state group (spearman 0.55 for IL-8 and 0.60 for MCP-1) (Table 2).
4. Discussion
The role of IL-8 and MCP-1 were evaluated in SCD patients. Of our young SCD patients, 22 (52.38%) were in crisis, and 20 (47.62%) were in a steady state status and represent our controls.
Circulating IL-8 levels were higher in steady state compared to crisis patients (1946 pg/mL+1384 versus 403.31 pg/mL+827.67, p=0.001). Among the SCD, the highest plasma levels of IL-8 were obtained with SAFA2 hemoglobin type (Sβ+Thalassemia). This may suggest that IL-8 could be used as a crisis biomarker as it is in other pathology [9] but further studies are required to consider any definitive conclusion.
In our study, the plasma levels of IL-8 were almost five–fold higher in controls patients than in patients with crisis (p=0.001). In Oman, a country in the southeastern coast of Arabian peninsula, Pathare showed that IL8 was also higher in controls than in crisis patients [10]. To explain this, some authors have shown that low circulating levels of IL-8 in SCD patients with crisis was associated with infection and dehydration [11, 12]. Unfortunately, no observations about infection or dehydration are available in our patients. Most people living with sickle cell disease in Africa have a clinical course more severe because of infectious, many others factors as nutritional factors [14] and a limited management of the disease [13].
On the other hand, IL8 was increased in both controls and VOC patients but higher in vasooclusive state [14–17]. Goncalves et al. think that genetic background should be involved with specific factors triggering crisis in the level of IL8 [14].
In our study, we focused our work on the plasma level of chemokines in our patients but according to Tam et al. [18], it is benefit to explore and compare, the level in plasma and the level in tissue where the inflammation takes place. They hypothesis that the systemic test may underestimate the concentrations at a local level. The authors recommend therefore that further studies should take in consideration both local and systemic aspects.
Inflammation is an evidence in sickle cell disease. In fact, at the local level where the inflammatory response lies, chemokines factors induce vasodilatation and recruitment of leukocytes, thereby establishing inflammatory reactions [19, 20]. Polynuclear neutrophils whose role is to defend the body was also high in our patients. Neutrophils are biomarkers of acute and chronic inflammation in sickle cell disease [20].
In our patients, high leucocytes and neutrophils count in particular was detected and showed increased circulating levels. White blood cells (WBC) mean and neutrophils mean count did not differ statistically between those with lower levels (?15pg/mL) of IL-8 (WBC: 12833+/-4599, neutrophils: 4806.31+/-3296.56) and high levels (WBC: 13109.37+/-4370.30, neutrophils: 6052.32+/-2520). WBC count did not differ according to the type of hemoglobin.
Red cells hemolysis trigger a sterile inflammatory reaction that stimulates neutrophils, immune first line of defense against infection and kill pathogen using many strategies, phagocytosis, secretion of antimicrobial proteins and formation of NETs (Neutrophils extracellular traps) [21].
Leukocytosis is almost constant in SCD patients and high leucocytes count is a factor of morbidity [22, 23], there are present in post-capillary veinules and slow down the circulation initiating vasoocclusive crisis [4].
We did not test CRP in our patients but many data are in favor of association of VOC frequency and increased level of Hs CRP support the need of testing CRP in VOC follow up [24].
We were curious to know if the Body Mass Index (BMI) was associated with selected chemokines. We classified patients in three groups: patients with overweight (19.05%), patients with underweight (42.86%) and patients with normal BMI (38.10%). Chemokines were not significantly different between the three groups and we concluded that the levels of cytokines IL-8 and MCP-1 were not correlated with BMI.
In contrast, Brunn and Kim found [25, 26], low IL-8 levels in overweight group compared to underweight and normal BMI and Brunn et al. demonstrated in vitro for the first time that adipose tissue may produce IL8 that could be involved in some obesity related complications.
Small number of patients enrolled is one limit in our study. The challenge in the future for a better care of SCD patients in crisis is important but before crisis is better. These information need to be checked in larger cohorts to confirm our findings.
Conclusion
Many evidences show the physiological role of cytokines in SCD. However, poor data are available for children with SCD in Sub-Saharan where the disease is most prevalent. Our data demonstrated that the levels of IL-8 and MCP-1 were significantly higher in steady state subjects compared with those in crisis. IL-8 and MCP-1 were positively correlated, supporting the idea that these chemokines are both implicated in inflammatory process in SCD. Finally, these chemokines can be biomarkers to phenotype SCD patients for both research and therapy with larger cohorts.
No Conflict of Interest
The authors declare that they have no conflict of interests.
Acknowledgements
The authors thank Biolegend staff in San Diego and especially Dr. Ji Shaoquan and Sun Binggang for having promptly accepted me in their laboratory for hands on training and graciously offered me the quantities of reagents needed for my work.
Reference
- Ebah LM. Renal involvement in sickle cell disease: an African perspective for an African condition. Clinical Kidney Journal 6 (2013): 6-7.
- Diallo D, Tchernia G. Sickle cell disease in Africa. Current Opinion in Hematology 9 (2002): 111-116.
- Williams TN. Sickle cell disease in sub-Saharan Africa. Hematology/Oncology Clinics 30 (2016): 343-358.
- WHO | Global epidemiology of haemoglobin disorders and derived service indicators. WHO, https://www.who.int/bulletin/volumes/86/6/06-036673/en/ (accessed 10 May 2020).
- Le DG, Lonsdorfer J, Fabritius H, et al. Prevalence of the sickle cell trait among students in a physical education college in Côte-d'Ivoire. Nouvelle Revue Francaise d'Hematologie 31 (1989): 409-412.
- Makis AC, Hatzimichael EC, Bourantas KL. The role of cytokines in sickle cell disease. Annals of Hematology 79 (2000): 407-413.
- Odièvre MH, Verger E, Silva-Pinto AC, et al. Pathophysiological insights in sickle cell disease. The Indian Journal of Medical Research 134 (2011): 532.
- Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine & Growth Factor Reviews 9 (1998): 9-23.
- Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, et al. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. European Cytokine Network 24 (2013): 106-113.
- Pathare A, Al Kindi S, Alnaqdy AA, et al. Cytokine profile of sickle cell disease in Oman. American Journal of Hematology 77 (2004): 323-328.
- Akodu SO, Diaku-Akinwumi IN, Njokanma OF. Obesity—does it occur in Nigerian children with sickle cell anemia. Pediatric Hematology and Oncology 29 (2012): 358-364.
- Duits AJ, Schnog JB, Lard LR, et al. Elevated IL?8 levels during sickle cell crisis. European Journal of Haematology 61 (1998): 302-305.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. The Lancet 376 (2010): 2018-2031.
- Gonçalves MD, Queiroz IL, Cardoso SA, et al. Interleukin 8 as a vaso-occlusive marker in Brazilian patients with sickle cell disease. Brazilian Journal of Medical and Biological Research 34 (2001): 1309-1313.
- Keikhaei B, Mohseni AR, Norouzirad R, et al. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. European Cytokine Network 24 (2013): 45-52.
- Pathare A, Kindi SA, Daar S, et al. Cytokines in sickle cell disease. Hematology 8 (2003): 329-337.
- Telen MJ. Biomarkers and recent advances in the management and therapy of sickle cell disease. F1000Research 4 (2015).
- Tam CS, Garnett SP, Cowell CT, et al. IL-6, IL-8 and IL-10 levels in healthy weight and overweight children. Hormone Research in Paediatrics 73 (2010):128-134.
- Platt OS. Sickle cell anemia as an inflammatory disease. The Journal of Clinical Investigation 106 (2000): 337-338.
- Torres LS, Okumura JV, Silva DG, et al. Inflammation in sickle cell disease: differential and down-expressed plasma levels of annexin A1 protein. PloS one 11 (2016).
- Gladwin MT, Ofori-Acquah SF. Erythroid DAMPs drive inflammation in SCD. Blood, The Journal of the American Society of Hematology 123 (2014): 3689-3690.
- Odièvre MH, Verger E, Silva-Pinto AC, et al. Pathophysiological insights in sickle cell disease. The Indian Journal of Medical Research 134 (2011): 532.
- Wun T, Cordoba M, Rangaswami A, et al. Activated monocytes and platelet?monocyte aggregates in patients with sickle cell disease. Clinical & Laboratory Haematology 24 (2002): 81-88.
- Mohammed FA, Mahdi N, Sater MA, et al. The relation of C-reactive protein to vasoocclusive crisis in children with sickle cell disease. Blood Cells, Molecules, and Diseases 45 (2010): 293-296.
- Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. The Journal of Clinical Endocrinology & Metabolism 86 (2001): 1267-1273.
- Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. International Journal of Obesity 30 (2006): 1347-1355.