Aortic Valve Insufficiency Related to Eosinophilic Granulomatosis with Polyangiitis
Article Information
Raphael Cauchois1*, Sebastien Renard2, Gilles Kaplanski1, Pierre-Andre Jarrot1
1Department of Internal Medicine and Clinical Immunology, CHU Conception, AP-HM, Aix Marseille Univ, C2VN, Marseille, France
2Department of Cardiology, CHU Timone, AP-HM, Aix Marseille Univ, Marseille, France
*Corresponding Author: Raphael Cauchois, Department of Internal Medicine and Clinical Immunology, CHU Conception, AP-HM, Aix Marseille Univ, C2VN, Marseille, France.
Received: 07 August 2022; Accepted: 20 August 2022; Published: 12 September 2022
Citation: Raphael Cauchois, Sebastien Renard, Gilles Kaplanski, Pierre-Andre Jarrot. Aortic Valve Insufficiency Related to Eosinophilic Granulomatosis with Polyangiitis. Archives of Clinical and Medical Case Reports 6 (2022): 624-625.
Share at FacebookKeywords
Eosinophilic granulomatosis; Aortic Valve; Surgery; Nasal polyposis
Article Details
Case Report
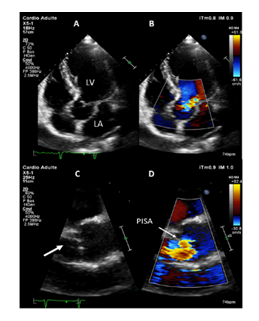
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare small-to-medium size vessel anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, which can display a broad spectrum of manifestations [1]. Although cardiac features are well-known in this disease, valvular insufficiency is rarely described. Herein, we describe a rare case of EGPA-related aortic insufficiency. A 62-year-old caucasian male with a previous history of surgery for nasal polyposis in 2011, was referred to our internal medicine department for a 3-week history of mild asthma, flu-like symptoms, 5 kilograms-weight loss, rhinosinusitis and electroneuromyography-confirmed mononeuritis multiplex affecting the left median and right peroneal nerves. On examination, besides previous evaluation, we were surprised by a marked diastolic murmur, which was maximal in the third left intercostal space, especially at the end of expiration suggesting important aortic insufficiency. Transthoracic echocardiography (Figure 1) showed normal systolic function, but aortic valvular thickening with partial right coronary cusp (RCC) prolapse leading to severe aortic insufficiency with no arguments for endocarditis on transesophageal echocardiography. Cardiac magnetic resonance imaging identified myocarditis with subepicardial enhancement of inferolateral wall associated with small pericardial effusion. Electrocardiogram was normal. The CT-scan revealed maxillary sinusitis, chest was normal. Laboratory findings revealed an elevated white blood cell count of 18.9G/L (N 4.0-11.0) with absolute eosinophil count of 8.80G/L (N 0.05-0.55), inflammatory syndrome (C-reactive protein 107mg/L (N < 5), fibrinogen 4.9g/L (N 1.80-4.0), elevated troponin (0.48ng/mL, N<0.05), BNP level (47pg/mL, N<100) and normal renal/liver functions. Microbiological samples (blood, urine cultures) were negative. Myeloperoxidase (MPO)-ANCA were detected by enzyme linked immunosorbent assay (128UI, N<20UI) yielded a diagnosis of EGPA. Considering the 2011 Five Factor score, this case of EGPA was severe and the patient was treated according to current recommendations [2]. The treatment combined corticosteroids (3 pulse of methylprednisolone followed with gradual taper of prednisone starting from 1mg/kg/day over 6 month) with 6 pulses of cyclophosphamide, relayed with azathioprine for 24 months. Patient achieved subsequent remission, and echocardiographic controls showed incomplete regression of aortic insufficiency after immunosuppressive therapy. Special attention has been paid to the prevention of complications from corticosteroids and immunosuppressants, in particular infectious events. Although the absence of pathological confirmation, the clinical evidence including the recent outbreak of asthma, non-lytic sinusitis, mononeuropathy multiplex, elevated eosinophils count associated with positive anti-MPO antibodies allowed us to retain the diagnosis of EGPA. After ruling out endocarditis by transesophageal echocardiography, aortic insufficiency was likely related to EGPA, since the patient had no previous history of aortic regurgitation on regular check-up before admission. Furthermore, the MRI-confirmed myocarditis and pericardial effusion suggesting a cardiac tropism of his vasculitis. Cardiac involvement, which represents 40% of cases [3], is rather associated with ANCA-negative phenotype and mainly characterized by myocarditis, pericarditis or endomyocardial fibrosis. This is the first cause of the EGPA-patients death, whose suspected mechanisms are vasculitis-related ischemia and eosinophilic infiltration of the myocardium [4,5]. Contrary to granulomatosis with polyangiitis (GPA, formerly called Wegener’s granulomatosis), 5 aortic valvular insufficiency was rarely described in EGPA. Only three additional cases of EGPA-related aortic insufficiency have been reported [6-8]. ANCA detection was negative in all cases, the diagnosis of EGPA was confirmed by pathological analysis of the required valve replacement revealing eosinophilic infiltrates, granuloma and necrosis on thickened leaflets. In addition to valve replacement, two patients achieved remission with corticosteroids, associated with oral cyclophosphamide in one case. The third died of cardiogenic shock before starting immunosuppressive drugs.
Once the diagnosis ANCA-associated vasculitis has been established, it is essential to conduct clinical and paraclinical exploration of the organs that may be affected, in order to adapt the intensity of the immunosuppressive treatment. International guidelines recommend systematic heart check-up by serum N-terminal pro-brain natriuretic peptide and troponin I measurements, chest imaging, electrocardiography and transthoracic echocardiography, increasing emphasis is however being placed on cardiac magnetic resonance, which could identify cardiac involvement in up to 62% of patients [9]. In conclusion, when faced with acute aortic insufficiency associated with systemic signs, infectious endocarditis must first be ruled out. Next, immune causes such as aortitis, non-infectious endocarditis or ANCA-associated vasculitis should be considered. Physicians should be aware of aortic insufficiency as a rare, but life-threatening complication of EGPA, and may perform a systematic cardiac evaluation in this disease. The treatment of EGPA with cardiac involvement is codified and should be initiated without delay.
Figure 1: Transthoracic echocardiography. (A-B) Transthoracic apical 5- chamber view (A) showing moderate to severe aortic regurgitation by color Doppler. (C-D) Transthoracic parasternal long axis view. Zoom of the aortic valve showing right coronary cusp prolapse (arrow, C) with moderate to severe aortic regurgitation by color Doppler (D) with obtention of a hemispheric PISA (proximal isovelocity surface area). LA: left atrium; LV: left ventricle.
References
- Kitching AR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers 71 (2020).
- Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med 26 (2015): 545-553.
- Sartorelli S, Chassagnon G, Cohen P, et al. Revisiting characteristics, treatment and outcome of cardiomyopathy in eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss). Rheumatology (Oxford) 61 (2022): 1175-1184.
- Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 65 (2013): 270-281.
- Lacoste C, Mansencal N, Ben m'rad M, et al. Valvular involvement in ANCA-associated systemic vasculitis: a case report and literature review. BMC Musculoskelet Disord 12 (2011): 50.
- Doherty L, Kumar P, Bexton R, et al. Aortic regurgitation and Churg-Strauss syndrome. QJM 98(2005): 772-773.
- Song YS, Seol S, Park J, et al. Large left ventricular non-infectious vegetation in patient with eosinophilic granulomatosis with polyangiitis. Cardiovasc J Afr 31 (2020): e1-e4.
- Karthikeyan K, Balla S, Alpert MA. Non-infectious aortic and mitral valve vegetations in a patient with eosinophilic granulomatosis with polyangiitis. BMJ Case Rep 12 (2019).
- Dennert RM, Paassen PV, Schalla S, et al. c. Arthritis Rheum 62 (2010): 627-634.