A Systematic Review of Vaccinations in Methotrexate Users
Article Information
Sheryl Mascarenhas1*
1Department of Internal Medicine, Division of Rheumatology, The Ohio State University Wexner Medical Center, 543 Taylor Ave, Columbus, OH 43203, USA
*Corresponding Author: Sheryl Mascarenhas, Department of Internal Medicine, Division of Rheumatology, The Ohio State University Wexner Medical Center, 543 Taylor Ave, Columbus, OH 43203, USA
Received: 25 June 2021; Accepted: 05 July 2021; Published: 13 July 2021
Citation:
Sheryl Mascarenhas, A Systematic Review of Vaccinations in Methotrexate Users. Fortune Journal of Rheumatology 3 (2021): 11-41.
Share at FacebookAbstract
With several COVID-19 vaccines now available, many questions regarding immunization within patients on immune modifying agents have been surfacing. One critical question is what effect methotrexate (MTX), a known inhibitor of antibody formation, may have on vaccine response during the pandemic. In particular, does holding methotrexate during the vaccination period have improved outcomes on vaccine response?
A systematic review was conducted of previous randomized controlled trials and clinical trials of vaccine studies for methotrexate use. Studies were limited to the adult population and to those with autoimmune rheumatic conditions. 29 studies were included for review. There was heterogeneity in vaccinations used including pneumococcal, influenza (H1N1, H3N2, and various B strains), tetanus toxoid, hepatitis A, and varicella zoster. Measurement of vaccine response was non-uniform among the studies. Methotrexate dosing in some studies was not reported, and in many studies was variable. 82.8% of the studies demonstrated methotrexate users were able to meet the study defined vaccine response in the majority of methotrexate users in at least 1 endpoint. Two studies examined vaccine disruption for influenza vaccines and demonstrated improved vaccine response to methotrexate users who discontinued therapy. Dosing of methotrexate was identified in 3 studies as having an impact on vaccine response.
Based off review of previous vaccine literature, a temporary hold of methotrexate in the post vaccination period may be a reasonable option to try boost the immune response to a novel vaccine.
Keywords
Methotrexate; Vaccine; Vaccination; COVID-19
Methotrexate articles; Vaccine articles; Vaccination articles; COVID-19 articles
Methotrexate articles Methotrexate Research articles Methotrexate review articles Methotrexate PubMed articles Methotrexate PubMed Central articles Methotrexate 2023 articles Methotrexate 2024 articles Methotrexate Scopus articles Methotrexate impact factor journals Methotrexate Scopus journals Methotrexate PubMed journals Methotrexate medical journals Methotrexate free journals Methotrexate best journals Methotrexate top journals Methotrexate free medical journals Methotrexate famous journals Methotrexate Google Scholar indexed journals Vaccine articles Vaccine Research articles Vaccine review articles Vaccine PubMed articles Vaccine PubMed Central articles Vaccine 2023 articles Vaccine 2024 articles Vaccine Scopus articles Vaccine impact factor journals Vaccine Scopus journals Vaccine PubMed journals Vaccine medical journals Vaccine free journals Vaccine best journals Vaccine top journals Vaccine free medical journals Vaccine famous journals Vaccine Google Scholar indexed journals Vaccination articles Vaccination Research articles Vaccination review articles Vaccination PubMed articles Vaccination PubMed Central articles Vaccination 2023 articles Vaccination 2024 articles Vaccination Scopus articles Vaccination impact factor journals Vaccination Scopus journals Vaccination PubMed journals Vaccination medical journals Vaccination free journals Vaccination best journals Vaccination top journals Vaccination free medical journals Vaccination famous journals Vaccination Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Rheumatology articles Rheumatology Research articles Rheumatology review articles Rheumatology PubMed articles Rheumatology PubMed Central articles Rheumatology 2023 articles Rheumatology 2024 articles Rheumatology Scopus articles Rheumatology impact factor journals Rheumatology Scopus journals Rheumatology PubMed journals Rheumatology medical journals Rheumatology free journals Rheumatology best journals Rheumatology top journals Rheumatology free medical journals Rheumatology famous journals Rheumatology Google Scholar indexed journals immunization articles immunization Research articles immunization review articles immunization PubMed articles immunization PubMed Central articles immunization 2023 articles immunization 2024 articles immunization Scopus articles immunization impact factor journals immunization Scopus journals immunization PubMed journals immunization medical journals immunization free journals immunization best journals immunization top journals immunization free medical journals immunization famous journals immunization Google Scholar indexed journals immune modifying agents articles immune modifying agents Research articles immune modifying agents review articles immune modifying agents PubMed articles immune modifying agents PubMed Central articles immune modifying agents 2023 articles immune modifying agents 2024 articles immune modifying agents Scopus articles immune modifying agents impact factor journals immune modifying agents Scopus journals immune modifying agents PubMed journals immune modifying agents medical journals immune modifying agents free journals immune modifying agents best journals immune modifying agents top journals immune modifying agents free medical journals immune modifying agents famous journals immune modifying agents Google Scholar indexed journals novel vaccine articles novel vaccine Research articles novel vaccine review articles novel vaccine PubMed articles novel vaccine PubMed Central articles novel vaccine 2023 articles novel vaccine 2024 articles novel vaccine Scopus articles novel vaccine impact factor journals novel vaccine Scopus journals novel vaccine PubMed journals novel vaccine medical journals novel vaccine free journals novel vaccine best journals novel vaccine top journals novel vaccine free medical journals novel vaccine famous journals novel vaccine Google Scholar indexed journals immune system articles immune system Research articles immune system review articles immune system PubMed articles immune system PubMed Central articles immune system 2023 articles immune system 2024 articles immune system Scopus articles immune system impact factor journals immune system Scopus journals immune system PubMed journals immune system medical journals immune system free journals immune system best journals immune system top journals immune system free medical journals immune system famous journals immune system Google Scholar indexed journals pneumococcal vaccines articles pneumococcal vaccines Research articles pneumococcal vaccines review articles pneumococcal vaccines PubMed articles pneumococcal vaccines PubMed Central articles pneumococcal vaccines 2023 articles pneumococcal vaccines 2024 articles pneumococcal vaccines Scopus articles pneumococcal vaccines impact factor journals pneumococcal vaccines Scopus journals pneumococcal vaccines PubMed journals pneumococcal vaccines medical journals pneumococcal vaccines free journals pneumococcal vaccines best journals pneumococcal vaccines top journals pneumococcal vaccines free medical journals pneumococcal vaccines famous journals pneumococcal vaccines Google Scholar indexed journals
Article Details
1. Introduction
COVID 19 has singularly changed the world with far reaching effects on nearly all aspects of life including health, families, relationships, religious worship, travel, economies, work, and politics. In November 2020, news broke of promising results from Pfizer’s COVID 19 vaccines. Soon Moderna, then later Johnson and Johnson followed suit, with others on the horizon. The news of these vaccines brought much needed hope for the world, but also many questions. One question of particular concern to rheumatologists and their patients was if the vaccine was effective in patients on medications that alter their immune system?
One of the most commonly used immune modifying agents in treating patients with autoimmune inflammatory conditions is methotrexate (MTX) [1]. According to the Centers for Disease Control and Prevention (CDC), MTX is immunosuppressive when it is administered at doses exceeding ≥0.4 mg/kg/week, whereas dosages below these levels may be considered as ‘low grade’ immunosuppressive [2]. MTX is a unique immune modifying agent as it has additional effects to not only treat the underlying autoimmune disease but also supplement biologic disease modifying anti-rheumatic drugs (DMARDs) by inhibiting antibody formation to these biologic agents [3]. This antibody inhibition has been further observed with MTX decreasing immunogenicity of various vaccines, including the seasonal influenza and pneumococcal vaccines [4].
In 2015 the American College of Rheumatology (ACR) released guidelines recommending vaccination in methotrexate users. These included pneumococcal, intramuscular influenza, hepatitis B (HB), human papilloma (HP), and live attenuated herpes zoster [5]. In 2019 the European League Against Rheumatism (EULAR) further supported administration of influenza, pneumococcal, tetanus toxoid, HB, hepatitis A virus (HAV), HP, vaccines to patients with autoimmune inflammatory rheumatic diseases (AIIRD) under immunosuppressive therapy, and consideration for use of herpes zoster [6]. In 2021 the American College of Rheumatology released guidelines which over the course of the pandemic have been updated. At the time of writing this article, the ACR most recently recommended for those with well controlled disease, holding MTX for 1 week after each of the 2mRNA vaccine doses; or holding for 2 weeks after single-dose COVID vaccine [7].
There have been a collection of studies evaluating immune response to various vaccines including pneumococcal, influenza, hepatitis A, and tetanus toxoid in patients on methotrexate. While none of these afore mentioned vaccines are RNA vaccines, like those being promulgated by Pfizer or Moderna, these studies offer insight into the immune response to patients on MTX and may lend additional guidance on adjustments that could be considered in managing patients on MTX.
2. Methods
The study design followed the statement on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [8]. A search strategy was developed using PUBMED databases with the MESH search terms of Vaccine or Vaccination and methotrexate with limits of clinical trial or randomized controlled trial. Studies involving MTX use for oncologic conditions, multiple sclerosis, and inflammatory bowel disease were excluded. Studies involving non-human subjects, or the pediatric population were excluded. Studies that clustered users and non-users of methotrexate into one group were excluded. However, studies that had subgroup analyses where select treatment arms combined methotrexate users and non-users but did identify discrete use in other arms were included with only the discrete use arms. Also excluded were select study arms where authors indicated they were including previously reported data in comparison to the current trial; this was done to eliminate duplication of data in the review. Reference lists for the selected studies from the initial database search were reviewed to identify further relevant papers. Figure 1 illustrates an overview of the search protocol.
Data was extracted independently and populated in a Microsoft Excel spreadsheet. A repeat data extraction was done on the selected studies eligible from the initial database search to confirm accuracy. Variables sought from these studies included type of study, study size, study arms, patient population (e.g. rheumatoid arthritis (RA), psoriatic arthritis (PsA), mean methotrexate dose, vaccine type, method for determining antibody response, and antibody response.
Risks of bias in this systematic review included publication bias and selective reporting in studies. To limit risk of bias, the study question of whether holding methotrexate or continuing methotrexate improved vaccine response was formulated prior to the data search. All studies meeting the above criteria were included in this review, regardless of the statistical significance of the results. To further limit journal publication bias recommendations from regulatory agencies including the ACR, EULAR, and the CDC were also reviewed. Selection bias is intrinsically limited in studies of this nature as blinding and allocation concealment have no impact on the purely objective outcome measure of interest in this review, serologic antibody response. Among the studies 93.1% of them administered a standard vaccine to all participants. One study did administer a placebo vaccine to subset of patients and one study administered either a single or two dose regimens of the influenza vaccine.
Immune response to evaluate vaccine efficacy may be measured by seroprotection, seroconversion, humoral and cell mediated response, and evaluation of the quality of produced antibodies [4]. Studies in this review commonly report IgG antibody titers, often accompanied by a measure of the increase in the antibody titer after vaccination, the geometric mean fold rise (GMFR). Typically, the post vaccine measurement occurs 3-6 weeks after vaccination, corresponding to when the peak IgG vaccine antibody is reached [9].
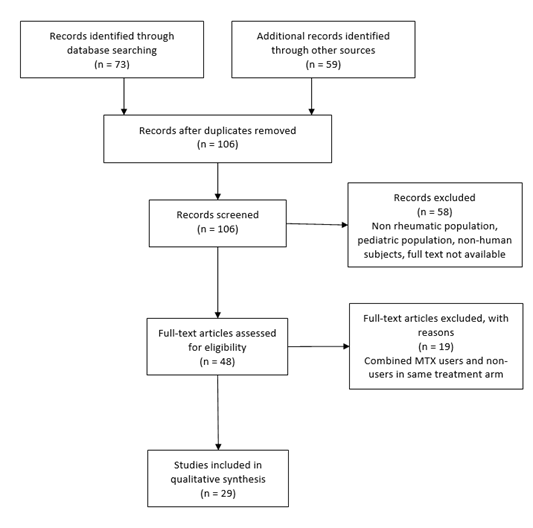
Figure. 1: Study methods.
MTX=methotrexate
3. Results
Per the protocol, 73 studies were amassed by using the study search terms on PubMed and applying limitations of clinical trials or randomized controlled trials. Studies with no human subjects, non-rheumatic disease populations, and pediatric populations were excluded. From the PubMed search 14 eligible studies were identified. The references from these publications were further reviewed for additional eligible studies. 59 studies were identified. After removing duplicates and applying the above exclusion criteria an additional 15 studies met protocol criteria to be added to the final qualitative analysis. In total 29 studies were included in the systematic review [10-38]. Supplemental Table includes the results of the data extraction from the final 29 studies.
Of note several studies included an arm that combined methotrexate users and non-users into one group. This was done when a patient was on a biologic DMARD with or without methotrexate. Rather than excluding the entire study, where possible the heterogeneous arms were excluded only. For example, one study included both MTX users and non-users in the abatacept (ABT) group, so this study arm was excluded in the review, however the MTX and control arms were included [29]. Similarly, another study grouped MTX users and non-users within the tocilizumab (TCZ) and adalimumab (ADM) arms hence both arms were excluded; however, the study did separate out rituximab (RTX) monotherapy from RTX taken in combination with MTX and so this subset of patients were included in the review [27]. The process of trying to preserve as much applicable data for the systematic review was also applied to two additional studies [33, 35].
4 Discussion
4.1 Does methotrexate decrease vaccine responses?
An assumption of this study design was that MTX inhibits immune response to vaccinations based off existing evidence that MTX impairs humoral response to pneumococcal vaccine. In a meta-analysis from 2014 MTX decreased pneumococcal response (OR 0.33 [95% CI 0.20–0.54] for serotype 6B and OR 0.58 [95% CI 0.36–0.94] for 23F) [4]. This is further demonstrated in studies included in this review. An outlying study was early work done by Kapetanovic MC’s group. This study suggested methotrexate actually seemed to improve immunogenicity for influenza vaccination, however this study did not include the mean or range doses of methotrexate for patients, a variable that may have significant effect on vaccine response as discussed later [22].
There are important practical points when reviewing these collective studies. While MTX did often result in lessened immunogenicity compared to either controls or anti-tumor necrosis factor (anti-TNF) therapies, there was generally sufficient immunity achieved for the majority of patients on MTX in at least 1 endpoint. The exceptions were demonstrated in 4 studies evaluating pneumococcal vaccine responses where 46%, 23%, 22.9%, and 18% of subjects achieved an adequate immune response to pneumococcal vaccine [12, 23, 25, 27]. The lone hepatitis A vaccine study in this review demonstrated abysmal results for methotrexate users with only 6% achieving a satisfactory immune response [36].
The practical point here is not that select studies had poor immune response, but rather that vaccinating on continuous methotrexate can still achieve an immune response in some patients. Therefore, if there are situations where a patient cannot disrupt methotrexate therapy, vaccination may still achieve reasonable immunity and be a better option than no vaccine at all. However, it lends itself to the ultimate question of this study. Does holding methotrexate around the time of vaccination improve antibody responses?
4.2 Does holding methotrexate improve vaccine response?
The most relevant data investigating this question comes out of a series of studies from Jin Kyun Park, et al. In 2017 their group conducted a multi-center trial evaluating whether holding methotrexate prior and/or after vaccination with quadrivalent seasonal influenza vaccine improved antibody responses. In this elegant study Park divided patients on MTX into 4 groups, continuing MTX, holding MTX 4 weeks pre-vaccination, holding MTX 2 weeks pre-vaccination and 2 weeks post-vaccination, and holding MTX 4 weeks post vaccination. All 4 groups mounted a satisfactory vaccine response to at least one influenza antigen, however the group which held MTX 2 weeks before and 2 weeks after the vaccination achieved greater vaccine response to at least two influenza antigens than continuous MTX users [21]. This difference was statistically significant when comparing the same groups’ response to all three antigens [21].
The robustness of the antibody response for the group holding MTX 2 weeks pre- and post-vaccination compared to continuous MTX use was evidenced further when the analysis was restricted to patients who lacked seroprotection before vaccination. With this subgroup analysis, holding MTX 2 weeks before and after vaccination led to statistically significant increases with all individual antibody titers when compared to continuous MTX use [21]. This may be especially of importance when considering pandemic vaccinations where the immune system for most of the population at large would not have encountered the virus.
Park’s research was the first to demonstrate that vaccine immunogenicity may be increased with vaccination occurring in the middle of a MTX discontinuation period. Additionally, the authors found that discontinuation after vaccination also achieved greater response compared to the continuous MTX users [21]. More specifically, the group that held methotrexate 4 weeks after vaccination also had significantly higher fold increases in antibody titers against H3N2 and B-Yamagata antigen and higher (though not statistically significant) increase in antibody titers against H1N1. Discontinuing MTX for 4 weeks after the vaccination was effective, although less effective than the group which discontinued 2 weeks before and 2 weeks post vaccination. This data suggests that MTX’s effects on immune cells is likely immediate [21].
In 2018, Park’s group investigated if a post vaccine disruption alone improved vaccine responses. In this study, subjects were randomized to either continue MTX or disrupt therapy for 2 weeks post vaccination of quadrivalent seasonal influenza. The authors found that holding MTX 2 weeks post vaccination versus continuous methotrexate had significant increases in immunogenicity to all 4 antigens (reported as % difference between holding methotrexate and continuing methotrexate (95% CI): H1N1: 11.9% (0.9% - 22.8%), p=0.033; H3N2: 16.8% (6.1%-27.4%), p=0.002; B-Yamagata: 22.7% (11.7% -33.7%), p<0.001; B-Victoria: 32.8% (21.8% -43.6%), p<0.001) [11]. Additionally, the MTX-hold group had significantly higher foldincreases in their antibody titers against all four influenza antigens [11].
4.3 Does the methotrexate dose matter?
In Park’s 2018 study, an analysis was performed on methotrexate dose effects. The authors reported no significant difference in vaccine response between the MTX-continuegroup and MTX-hold group for those who took MTX 7.5 mg or less per week [11]. However the difference was significant when comparing those on MTX 15 mg or more per week [11]. Park’s study suggests that vaccine dose may have significant effect on immunogenicity. Other studies in this review noted a dose effect on methotrexate immunogenicity. While the exact dosing is not reported, Ribero AC, at al. note average/high dosing of methotrexate resulted in decreased immunogenicity [13]. Bingham CO III, et al. also found MTX dose to be a predictor of immunogenicity [16].
The outlying study however is Kaine JL, et al, where patients were divided into 4 arms, placebo, MTX, adalimumab (ADM), or ADM+MTX. The authors did a sub-analysis based on MTX dosing. They divided the MTX users into 3 dose groups which included: >0-10 mg/week, >10-15 mg/week, and > 15 mg/week. The different dose groups did not follow the patterns seen by Park; however interpretation of this study is limited due to the small sample size [26]. Additionally, this study grouped patients who were either above or below 7.5 mg into the same group. Recall the Park 2018 study noted the difference occurring with MTX dose was at or below 7.5 mg/week [11].
BAFF inhibition of immunogenicity has been implicated for the disparity among MTX users. In the 2018 Park study, among patients who continued MTX, vaccine responders had significantly lower BAFF levels than the non-responders to ≥2/4 antigens [11]. However, in patients who held methotrexate, BAFF levels did not differ significantly between vaccine responders and non-responders [11]. Further, antibody titer changes for pre and post vaccination for individual antigens H1N1, B Yamagata and B Victoria correlated inversely with serum BAFF levels in the MTX-continue group but not in the MTX-hold group (p=0.047, p=0.019, p=0.045, respectively) [11]. There was however no statistical significance for H3N2 (p=0.177) [11]. The inverse correlation between BAFF levels and antibody production seemed to be more robust in patients taking MTX>15 mg/week than those taking MTX<7.5 mg/week [11].
There appears to be some correlation with methotrexate dosing and immunogenicity. Interestingly review of the listed studies in the Supplemental Table demonstrates many do not list the methotrexate dose or that most hover around the 15 mg dose of methotrexate. This may suggest that much of the data that exists about vaccination in methotrexate users may be limited as methotrexate use has generally been considered all or none rather than dose dependent in most of the previous vaccine studies.
Further studies should be undertaken specifically evaluating the effects of methotrexate dosing on vaccine response. However, during this pandemic vaccination where time for such a study is not possible, based off the limited observations from the above studies it may be worth recommending methotrexate users on moderate to high doses more heavily consider methotrexate disruption. Potentially low dose methotrexate users may be able to continue therapy throughout the vaccination period.
4.4 Does the number of vaccine doses matter?
Talk of booster doses is already being suggested for healthy individuals, however some have already looked at boosters in methotrexate users with previous vaccines. One of the reviewed studies evaluated whether additional booster doses may be more beneficial to immunocompromised patients. This study originated in the time of a looming pandemic with influenza A (H1N1) in 2009. At that time, the outbreak led to rapid development of novel influenza vaccines that were distributed and administered globally to hundreds of millions within a few months [39]. The same questions we are asking now about improving vaccine response were being asked then in 2009. In efforts to improve vaccine responses, the medical community in Switzerland administered a novel regimen where adjuvant influenza vaccines were administered in a 2-dose schedule to immunocompromised patients and 1 dose for healthy individuals for H1N1 [39]. Booster dosing is not a novel concept, in fact the CDC recommends consideration of an additional dose of hepatitis A vaccine in patients on anti-TNF agents and/or MTX [1].
Gabay C, et al. assessed primary and secondary vaccine responses to these novel adjuvant influenza vaccines in Switzerland between immunocompromised and healthy individuals. In Gabay’s study there were 72 of 173 patients in total on MTX with the remainder on other DMARDs or immunosuppressive agents. After the first vaccine dose antibody responses were significantly lower in immunocompromised patients (GMT 146 versus 340, p < 0.001, seroprotection rate 74.6% versus 87%, p < 0.001) [39]. However, these differences became statistically insignificant after the immunocompromised group received the second vaccination when compared to the healthy controls who received 1 vaccination [39]. Key inhibitors of vaccine immunogenicity in this study included increasing age, DMARDs (except hydroxychloroquine, sulfasalazine, and tumor necrosis factor α antagonist treatment), and recent (within 3 months) B cell depletion treatment [39].
Gabay’s study raises important implications for a standard 2 dose RNA vaccine. Both Moderna and Pfizer vaccines are administered as 2 dose vaccines routinely. Whether immunogenicity would be improved among immunocompromised patients with additional doses would be important to further elucidate.
5. Conclusion
A 2-week disruption of MTX as previously studied may be tenable for some well controlled patients and may possibly lead to improved immune responses to vaccinations. Given that methotrexate effects on immune cells appears to be immediate, holding methotrexate after vaccination may further improve immune response.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Li P, Zheng Y, Chen X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics.Front Pharmacol 8 (2017): 460.
- Day AL, Winthrop KL, Curtis JR. The effect of disease modifying anti-rheumatic drugs on vaccine immunogenicity in adults. Cleveland Clinic Journal of Medicine 87 (2020): 695-703.
- Jani M, Barton A, Warren RB, et al. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases.Rheumatology (Oxford) 53 (2014): 213-222.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 66 (2014): 1016-1026.
- Singh JA, Saag KG, Bridges SL Jr, et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 68 (2016): 1-26.
- FurerV,RondaanC,HeijstekMW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases Annals of the Rheumatic Diseases79 (2020): 39-52.
- Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology Guidance for COVID 19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases: Version 1. Arthritis Rheumatol (2021).
- PRISMA transparent reporting of systematic reviews and meta-analysis. PRISMA website. Updated 2015. Accessed November 29 (2020).
- Siegrist CA.Vaccine immunology. In:S Plotkin,W Orenstein,P Offit, editors.Vaccines.6th ed.Philadelphia: W.B. Saunders Company (2012): p.17-36
- Winthrop KL, Wouters AG, Choy EH, et al. The Safety and Immunogenicity of Live Zoster Vaccination in Patients with Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial.Arthritis Rheumatol 69 (2017): 1969-1977.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial.Ann Rheum Dis 77 (2018): 898-904.
- Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis.Ann Rheum Dis 75 (2016): 687-695.
- Ribeiro AC, Guedes LK, Moraes JC, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice.Ann Rheum Dis 70 (2011): 2144-2147.
- Kapetanovic MC, Kristensen LE, Saxne T, et al. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis.Arthritis Res Ther16 (2014): R2
- Kapetanovic MC, Saxne T, Sjoholm A, et al. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis.Rheumatology (Oxford)45 (2006): 106-111.
- Bingham CO III, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial.Arthritis Rheum62 (2010): 64-74.
- Mori S, Ueki Y, Akeda Y, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy.Ann Rheum Dis 72 (2013): 1362-1366.
- Mori S, Ueki Y, Hirakata N, et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis.Ann Rheum Dis71 (2012): 2006-2010.
- Bingham CO III, Rizzo W, Kivitz A, et al.Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA).Ann Rheum Dis74 (2015): 818-822.
- Calabrese LH, Abud-Mendoza C, Lindsey SM, et al. Live Zoster Vaccine in Patients with Rheumatoid Arthritis Treated with Tofacitinib with or Without Methotrexate, or Adalimumab with Methotrexate: A Post Hoc Analysis of Data From a Phase IIIb/IV Randomized Study.Arthritis Care Res (Hoboken) 72 (2020): 353-359.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial.Ann Rheum Dis 76 (2017): 1559-1565.
- Kapetanovic MC, Saxne T, Nilsson JA, et al. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology 46 (2007): 608-611.
- Park JK, Lee YJ, Bitoun S, et al. Interaction between B-cell activation factor and methotrexate impacts immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis.Ann Rheum Dis 78 (2019): 282-284.
- Visvanathan S, Keenan GF, Baker DG, Levinson AI, Wagner CL. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone.J Rheumatol 34 (2007): 952-957.
- Mease P, Ritchlin C, Martin R, et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol 31 (2004): 1356-1361.
- Kaine J, Kivitz A, Birbara C, et al. Effect of adalimumab (Humira®) on response to pneumococcal and influenza virus vaccines in patients with rheumatoid arthritis (RA). Ann Rheum Dis 65 (2006): Suppl II-304.
- Crnkic Kapetanovic M, Saxne T, Jönsson G, et al. Rituximab and abatacept but not tocilizumab impairs antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis.Arthritis Res Ther 15 (2013): R171.
- Kapetanovic MC, Roseman C, Jönsson G, et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 63 (2011): 3723-3732.
- Ribeiro AC, Laurindo IM, Guedes LK, et al. Abatacept severely reduces the immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis.Arthritis Care Res (Hoboken)15 (2013): 476-480.
- O'Dell J,Gilg J,Palmer W,et al.Pneumococcal vaccine in rheumatoid arthritis: decreased response while on methotrexate.J Clin Rheumatol 2 (1996):59-63.
- Migita K, Akeda Y, Akazawa M, et al. Opsonic and Antibody Responses to Pneumococcal Polysaccharide in Rheumatoid Arthritis Patients Receiving Golimumab Plus Methotrexate [published correction appears in Medicine (Baltimore).95 (2016): e8362. Oish, Kazunori [Corrected to Oishi, Kazunori]].Medicine (Baltimore) 94 (2015): e2184.
- Kivitz AJ, Schechtman J, Texter M, et al.Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial.J Rheumatol41 (2014): 648-657.
- Kobie JJ, Zheng B, Bryk P, et al.Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor.Arthritis Res Ther13 (2011): R209.
- Crnkic Kapetanovic M, Saxne T, Truedsson L, et al. Persistence of antibody response 1.5 years after vaccination using 7-valent pneumococcal conjugate vaccine in patients with arthritis treated with different antirheumatic drugs.Arthritis Res Ther 15 (2013): R1.
- Migita K, Akeda Y, Akazawa M, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus.Arthritis Res Ther 17 (2015): 149.
- Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study.Travel Med Infect Dis 12 (2014): 134-142.
- Migita K, Akeda Y, Akazawa M, et al. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res Ther 17 (2015): 357.
- Bingham CO 3rd, Winthrop KL, Yang L, et al. BAFF inhibition does not significantly impair immunization responses in patients with rheumatoid arthritis.Arthritis Res Ther 17 (2015): 347.
- Gabay C, Bel M, Combescure C, et al.Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study.Arthritis Rheum63 (2011): 1486-1496.
Supplementary Material
SD=Standard deviation; Ref=reference; RCT=randomized controlled trial; MTX =methotrexate; TOF=tofacitinib; VZV=varicella zoster vaccine; GMFR=geometric mean fold rise; cont=continue; HIA=haemagglutination inhibition antibody; vac=vaccination; mono=monotherapy; SCR=seroconversion rate; SPR=seroprotection rate; DMARD=disease modifying anti-rheumatic drug; SC=seroconversion; SR=seroprotection; LTE=long-term extension study; CT=clinical trial; GMT=geometric mean titer; TNF=anti-tumor necrosis factor; RTX=rituximab; KLH=keyhole limpet hemocyanin; TCZ=tocilizumab; IQR=interquartile range; GMC=geometric mean concentration; OI= opsonisation indices; GM=geometric mean; GM-OI=geometric mean opsonisation index; ADM=adalimumab; IR=incidence rate; INX=infliximab; N/A=not applicable; ETC=etanercept; NSAID=non-steroidal anti-inflammatory drug; ARR=antibody response ratio; RA=rheumatoid arthritis; FI=factor increase; ABT=abatacept; GOM=golimumab; OPA= opsonophagocytic activity; CZP=certolizumab pegol; SpA=spondyloarthropathy; TAC=tacrolimus; HAV=hepatitis A virus, Q=every; W=week, TAB=tabalumab
