A Randomized Controlled Clinical Trial to Study the Impact of Multigrain Instant Health Drink Mix in Clinical Management of Anemia in Women
Article Information
Vidyadhar Vaidya*, Gayatri Ganu, Ninad Naik, Murugan Narayanaswamy
Mprex Healthcare P. Ltd, Crossroad, 501, Bhumkar Bridge, Wakad, Pune, Maharashtra 411057, India
*Corresponding Author: Mprex Healthcare Pvt. Ltd, Crossroad, 501, Bhumkar Bridge, Wakad, Pune, Maharashtra 411057, India
Received: 19 March 2022; Accepted: 29 March 2022; Published: 11 April 2022
Citation: Vidyadhar Vaidya, Gayatri Ganu, Ninad Naik, Murugan Narayanaswamy. A randomized controlled clinical trial to study the impact of Multigrain Instant Health Drink Mix in clinical management of anemia in women. Journal of Food Science and Nutrition Research 5 (2022): 452-469.
Share at FacebookAbstract
Background
WHO considers Iron Deficiency Anemia (IDA) as one of the most expensive diseases in the world due to lost productivity and the sheer numbers of the affected population. In 2019, global anemia prevalence was 29.9% (95% uncertainty interval (UI) 27.0%, 32.8%) in women of reproductive age, equivalent to over half a billion women aged 15-49 years. Prevalence was 29.6% (95% UI 26.6%, 32.5%) in non-pregnant women of reproductive age, and 36.5% (95% UI 34.0%, 39.1%) in pregnant women.
Trial design
Trial was randomized controlled and parallel arm involving 60 patients with Anemia included in two parallel groups, Health Drink Mix Group and Control Group (n = 30/group).
Methodology
Health drink mix group (N=30) were advised to take 30 gm Multigrain Instant Health Drink Mix with warm milk twice a day for 90 days and were provided counselling for nutrition and anemia over 90 days. Control group (N=30) were advised to consume warm milk twice a day and provided counselling for nutrition and anemia over 90 days.
Results
There was significant increase in hemoglobin levels (20.6%) in Health Drink Mix treated group compared to 2.76% in control group. The levels of serum iron were significantly increased in Health Drink Mix Group than control group (35.38%). Health Drink Mix led to reduction in symptoms such as weakness, pale and yellow skin, dysmenorrhea, lightheadedness. There was significant reduction in fatigue severity score in Health Drink Mix treated group than control.
Conclusion
It can be concluded from the present study that
Keywords
Anemia, Health drink mix, Serum iron, Iron deficiency anemia
Anemia articles; Health drink mix articles; Serum iron articles; Iron deficiency anemia articles
Anemia articles Anemia Research articles Anemia review articles Anemia PubMed articles Anemia PubMed Central articles Anemia 2023 articles Anemia 2024 articles Anemia Scopus articles Anemia impact factor journals Anemia Scopus journals Anemia PubMed journals Anemia medical journals Anemia free journals Anemia best journals Anemia top journals Anemia free medical journals Anemia famous journals Anemia Google Scholar indexed journals Health drink mix articles Health drink mix Research articles Health drink mix review articles Health drink mix PubMed articles Health drink mix PubMed Central articles Health drink mix 2023 articles Health drink mix 2024 articles Health drink mix Scopus articles Health drink mix impact factor journals Health drink mix Scopus journals Health drink mix PubMed journals Health drink mix medical journals Health drink mix free journals Health drink mix best journals Health drink mix top journals Health drink mix free medical journals Health drink mix famous journals Health drink mix Google Scholar indexed journals Serum iron articles Serum iron Research articles Serum iron review articles Serum iron PubMed articles Serum iron PubMed Central articles Serum iron 2023 articles Serum iron 2024 articles Serum iron Scopus articles Serum iron impact factor journals Serum iron Scopus journals Serum iron PubMed journals Serum iron medical journals Serum iron free journals Serum iron best journals Serum iron top journals Serum iron free medical journals Serum iron famous journals Serum iron Google Scholar indexed journals Iron deficiency anemia articles Iron deficiency anemia Research articles Iron deficiency anemia review articles Iron deficiency anemia PubMed articles Iron deficiency anemia PubMed Central articles Iron deficiency anemia 2023 articles Iron deficiency anemia 2024 articles Iron deficiency anemia Scopus articles Iron deficiency anemia impact factor journals Iron deficiency anemia Scopus journals Iron deficiency anemia PubMed journals Iron deficiency anemia medical journals Iron deficiency anemia free journals Iron deficiency anemia best journals Iron deficiency anemia top journals Iron deficiency anemia free medical journals Iron deficiency anemia famous journals Iron deficiency anemia Google Scholar indexed journals blood hemoglobin articles blood hemoglobin Research articles blood hemoglobin review articles blood hemoglobin PubMed articles blood hemoglobin PubMed Central articles blood hemoglobin 2023 articles blood hemoglobin 2024 articles blood hemoglobin Scopus articles blood hemoglobin impact factor journals blood hemoglobin Scopus journals blood hemoglobin PubMed journals blood hemoglobin medical journals blood hemoglobin free journals blood hemoglobin best journals blood hemoglobin top journals blood hemoglobin free medical journals blood hemoglobin famous journals blood hemoglobin Google Scholar indexed journals Health Drink Mix articles Health Drink Mix Research articles Health Drink Mix review articles Health Drink Mix PubMed articles Health Drink Mix PubMed Central articles Health Drink Mix 2023 articles Health Drink Mix 2024 articles Health Drink Mix Scopus articles Health Drink Mix impact factor journals Health Drink Mix Scopus journals Health Drink Mix PubMed journals Health Drink Mix medical journals Health Drink Mix free journals Health Drink Mix best journals Health Drink Mix top journals Health Drink Mix free medical journals Health Drink Mix famous journals Health Drink Mix Google Scholar indexed journals vitamin B9 articles vitamin B9 Research articles vitamin B9 review articles vitamin B9 PubMed articles vitamin B9 PubMed Central articles vitamin B9 2023 articles vitamin B9 2024 articles vitamin B9 Scopus articles vitamin B9 impact factor journals vitamin B9 Scopus journals vitamin B9 PubMed journals vitamin B9 medical journals vitamin B9 free journals vitamin B9 best journals vitamin B9 top journals vitamin B9 free medical journals vitamin B9 famous journals vitamin B9 Google Scholar indexed journals vitamin B12 articles vitamin B12 Research articles vitamin B12 review articles vitamin B12 PubMed articles vitamin B12 PubMed Central articles vitamin B12 2023 articles vitamin B12 2024 articles vitamin B12 Scopus articles vitamin B12 impact factor journals vitamin B12 Scopus journals vitamin B12 PubMed journals vitamin B12 medical journals vitamin B12 free journals vitamin B12 best journals vitamin B12 top journals vitamin B12 free medical journals vitamin B12 famous journals vitamin B12 Google Scholar indexed journals diabetes articles diabetes Research articles diabetes review articles diabetes PubMed articles diabetes PubMed Central articles diabetes 2023 articles diabetes 2024 articles diabetes Scopus articles diabetes impact factor journals diabetes Scopus journals diabetes PubMed journals diabetes medical journals diabetes free journals diabetes best journals diabetes top journals diabetes free medical journals diabetes famous journals diabetes Google Scholar indexed journals
Article Details
1. Introduction
Anemia is defined as a decreased concentration of blood hemoglobin. It is a condition in which the number of red blood cells or their oxygen-carrying capacity is insufficient to meet the body's physiological requirements, which vary by age, sex, altitude, smoking habits, and during pregnancy [1]. In 2019, global anemia prevalence was 29.9% (95% uncertainty interval (UI) 27.0%, 32.8%) in women of reproductive age. Prevalence was 29.6% (95% UI 26.6%, 32.5%) in non-pregnant women of reproductive age, and 36.5% (95% UI 34.0%, 39.1%) in pregnant women [2]. WHO considers Iron Deficiency Anemia (IDA) as one of the most expensive diseases in the world due to lost productivity and the sheer numbers of the affected population. First-line treatment is oral therapy with ferrous iron salts, but a substantial proportion of patients suffer from gastrointestinal side-effects, resulting in non-adherence and treatment failure. Gastrointestinal symptoms most probably result from a combination of two factors: (i) free radical generation through iron-induced redox cycling in the gut lumen and at the mucosal surface which can promote inflammation and (ii) changes to the microbiota composition or metabolism [3]. It is need of the time to look at the anemia from nutritional correction point of view and have a strategy in place to improve the anemia markers like Hb, iron etc. with incorporating nutritional factors for achieving most safe, efficacious and sustainable treatment of anemia. Multigrain Instant health drink mix for Women manufactured by Southern Nutrition Pvt. Ltd., possess novel Iron lock technology. The formulation provides 100% RDA of Iron, vitamin C, vitamin B9 & B12 in 2 servings of 30 gm each. The product is a unique mix of different millets, vitamins, iron, calcium and other micronutrients. The current study is undertaken, considering the novel composition and need of intervention for management of anemia in women.
Study objectives
The primary objectives of the study were to assess the effectiveness of Multigrain Instant health drink mix for Women by evaluating iron deficiency parameters like hemoglobin, serum iron, serum ferritin and total iron binding protein level from baseline to day 90 between groups. The secondary objectives of the study were to assess improvement in quality of life along with the symptoms of the anemia. The tolerability and safety was also evaluated.
Inclusion criteria
Females of age group between 18-45 (Both inclusive) visiting outpatient department of study site were considered for the study. Those willing to give written informed consent and willing to adhere to protocol requirements were selected. The criteria of selection of subjects with anemia for the present study was with or without folate/ vitamin B12 deficiency having hemoglobin concentration less than 12 g/dL but more than 8 g/dL with or without symptoms of anemia.
Exclusion criteria
The subjects getting any iron supplements for treating anemia, or consuming any complementary medicine, dietary supplements containing iron, vitamins, antioxidants etc were not considered for the study. Subjects who participated in clinical trial for anemia in last 3 months’ span of screening were not eligible for the inclusion. Vulnerable group like pregnant and breastfeeding women were strictly excluded from study. Subjects with known history of hypersensitivity for the investigational product ingredients, with or without PCOD, diabetes, hypertension, thyroid disorder or other fertility disorders which proved unfit from investigator viewpoint were excluded. Subjects on hormone replacement therapy and/or taking contraceptive pills for birth control, having medical history of malabsorption syndrome, hemochromatosis and with obvious internal or external bleeding as documented by medical history or any surgeries undergone within past 4 weeks were not considered for the study participation.
2. Methodology
We conducted a prospective randomized controlled trial involving anemia patients recruited from the outpatient department of Lokmanya Medical Research Centre, Lokmanya Hospital, Chinchwad, Pune, India. The study was approved by the Institutional Ethics Committee, Lokmanya Medical Research Centre, and was registered with the Clinical Trial Registry of India (CTRI/2021/07/034881). The consolidated standards of reporting trials (CONSORT) flow of the entire study is depicted in figure 1. The informed consent from every subject participated in study was obtained before screening. In the current randomized controlled trial, 60 patients with Anemia were included in two parallel groups (n = 30/group). Subjects in group A i.e. Health Drink Mix group (N=30) were advised to take 30 gm of powder with warm milk twice a day for 90 days and were provided counselling for nutrition and anemia over 90 days. Subjects in group B i.e. Control group (N=30) were advised to consume warm milk twice a day and provided counselling for nutrition and anemia over 90 days. The follow up schedule was on every month for 3 months. On each visit day, subject underwent clinical examination, screening for any adverse event and blood sample collection for complete blood count and serum iron, ferritin and TIBC. On baseline and day 90 visit all subjects were assessed for quality of life, changes in symptoms, CLBT score, energy audit questionnaire and fatigue score etc.
Figure 1: Consort Chart
Intervention
Instant Health Drink Mix powder contained Milk solids 29%, Grains 20% [Millets – 7.3%: Bajra, Sorghum, Ragi; Cereals – 6.7%: Maize Wheat; Super grains - 6%: Quinoa, Amaranth, Black rice], Sugar, Maltodextrin, Malt extract, Cocoa powder, Minerals, Stabilizers (INS 466, INS 415), Vitamins, Steviol glycosides (E960), extracted from Steviol leaf.
Sample size
We wish to enroll more than 60 subjects to get 60 evaluable cases. We have considered around 5% increase in hemoglobin levels in Instant Health Drink Mix group than control. Based on this assumption from clinical experience, a qualified statistician evaluated the sample size of total 60 (30 cases in each arm) completed cases needed to assess the study objective at 90% power and alpha 0.05.
Randomization
We screened 70 participants based on the above-mentioned inclusion/exclusion criteria, of which 60 participants were found suitable for inclusion in the study. They were randomized using a computer-generated randomization sheet to receive either in the treatment group with Health Drink Mix or Control group. There were no drop outs in trial. Figure 1 presents the flow of events for the trial. Mechanism used to implement the random allocation sequence was sequentially numbered containers, as the trial is open label there is no blinding. We received randomization schedule from qualified statistician, investigator enrolled the participants to respective study groups.
Statistical Analysis
Patients without any major protocol violation were included in the per-protocol population (pp), including those who had good treatment compliance, and those who did not take any prohibited medications during the study period with completed CRF. Both descriptive and inferential analyses were used for inferring the data. All p-values were reported based on a two-sided significance test and all the statistical tests were interpreted at a 5% level of significance. Continuous variables, such as age and other demographical characteristics, were summarized by using summary statistics, i.e. frequency, and mean, and standard deviation. Hematological, biochemical and scores along with vitals are analyzed by student t test.
3. Results
Demographic characteristics
The sample size of this study was 60, i.e., 30 participants in each group; however, all 60 participants completed the study [n=30 in the Health Drink Mix (Treatment group); n=30 in Control group]. There were no drop out of patients from the study (Figure 1). In the present study the mean age of female subjects in test and control group were comparable and ranged from 18 to 45 years. The details are presented in table 1.
|
Parameter |
Health Drink Mix Group (N=30) |
Control Group (N=30) |
|
Age (years) |
35±8 |
36±7 |
|
Age Range (years) |
18.00 to 45.00 years |
18.00 to 45.00 years |
By Student t test NS = Not Significant
Table 1: Demographic details of study subjects
Changes in mean hemoglobin levels between groups
At baseline the hemoglobin levels in both Health Drink Mix and control group were comparable. At day 30, 60 and 90 there was significant increase in hemoglobin levels in Health Drink Mix treated group compared to control group (table 2). The percent increase in hemoglobin level was 20.6% in Health Drink Mix treated group compared to 2.76% in control group (Figure 2).
|
Duration |
Hemoglobin (g/dL) (mean ± SD) |
||
|
Health Drink Mix Group |
Control Group |
P value |
|
|
Baseline |
10.15±1.19 |
10.48±1.32 |
0.318 |
|
Day 30 |
11.69±1.29 |
10.79±1.28 |
|
|
Day 60 |
12.21±1.17 |
10.75±1.36 |
|
|
Day 90 |
12.24±1.17 |
10.77±1.37 |
|
|
Mean diff (Baseline – Day 30) |
-1.54±0.91 |
-0.31±0.29 |
<0.001 |
|
Mean diff (Baseline – Day 60) |
-2.05±0.97 |
-0.27±0.39 |
<0.001 |
|
Mean diff (Baseline – Day 90) |
-2.09±0.96 |
-0.29±0.38 |
<0.001 |
Data analyzed by student t test. Significant at p<0.05.
Table 2: Changes in mean hemoglobin levels between groups
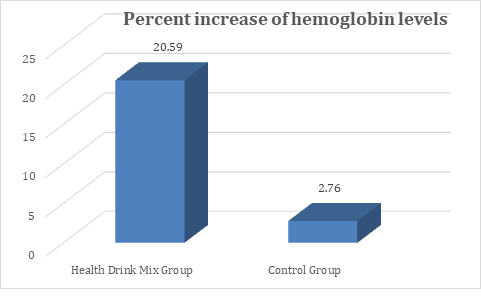
Figure 2: Changes in percent increase in mean hemoglobin levels between groups
Changes in mean serum Ferritin levels between groups
In the present study, at baseline the mean ferritin levels were comparable between groups and were recorded as 54.11 and 51.39 ng/ml in Health Drink Mix and control group respectively. After treatment at day 30, 60 and 90 the levels of ferritin were significantly increased in Health Drink Mix Group than control group (table 3). The percent increase of ferritin levels in Health Drink Mix Group was 16.33% compared to almost negligible increase in control group (Figure 3).
|
Duration |
Serum Ferritin (ng/ml) (mean ± SD) |
||
|
Health Drink Mix Group |
Control Group |
P value |
|
|
Baseline |
54.11±45.94 |
51.39±36.20 |
0.8 |
|
Day 30 |
60.58±46.21 |
52.71±36.15 |
|
|
Day 60 |
62.51±46.04 |
53.30±35.52 |
|
|
Day 90 |
62.95±45.82 |
51.45±34.48 |
|
|
Mean diff (Baseline – Day 30) |
-6.47±4.23 |
-1.32±1.25 |
<0.0001 |
|
Mean diff (Baseline – Day 60) |
-8.40±5.89 |
-1.91±1.67 |
<0.0001 |
|
Mean diff (Baseline – Day 90) |
-8.84±6.11 |
-0.06±11.66 |
0.001 |
Data analyzed by student t test. Significant at p<0.05.
Table 3: Changes in mean serum Ferritin levels between groups
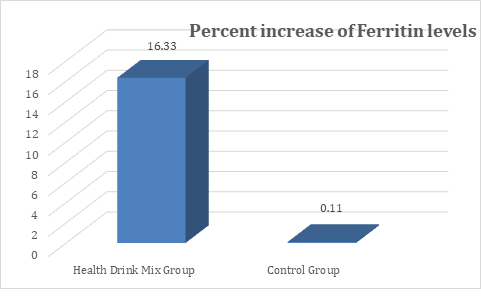
Figure 3: Changes in percent increase in mean serum ferritin levels between groups
Changes in mean serum iron levels between groups
In the present study, at baseline the mean serum iron levels were comparable between groups and were recorded as 50.67 and 41.13 µg/dL in Health Drink Mix and control group respectively. After treatment at day 30, 60 and 90 the levels of serum iron were significantly increased in Health Drink Mix Group than control group (table 4). The percent increase of iron levels in Health Drink Mix Group was 35.38% compared to 8.31% increase in control group (Figure 4).
|
Duration |
Serum Iron (µg/dL) (mean ± SD) |
||
|
Health Drink Mix Group |
Control Group |
P value |
|
|
Baseline |
50.67±31.64 |
41.13±30.87 |
0.242 |
|
Day 30 |
58.97±30.27 |
43.30±31.43 |
|
|
Day 60 |
64.37±30.11 |
44.10±31.27 |
|
|
Day 90 |
70.60±30.07 |
44.55±31.23 |
|
|
Mean diff |
-8.30±4.09 |
-2.16±3.48 |
<0.0001 |
|
(Baseline – Day 30) |
|||
|
Mean diff |
-13.70±6.98 |
-2.96±3.60 |
<0.0001 |
|
(Baseline – Day 60) |
|||
|
Mean diff |
-19.93±7.26 |
-3.42±4.01 |
<0.0001 |
|
(Baseline – Day 90) |
|||
Data analyzed by student t test. Significant at p<0.05.
Table 4: Changes in mean serum iron levels between groups
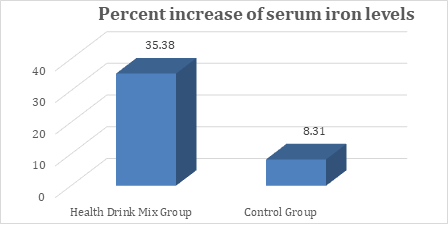
Figure 4: Changes in percent increase in mean serum iron levels between groups
Changes in mean total iron binding protein (TIBC) levels between groups
In the present study, at baseline the mean TIBC levels were comparable between groups and were recorded as 399.05 and 381.41µg/dL in Health Drink Mix and control group respectively. After treatment at day 30, 60 and 90 the levels of TIBC were significantly increased in Health Drink Mix Group than control group (table 5). The percent increase of TIBC levels in Health Drink Mix Group was 3.58% compared to 0.87% increase in control group (Figure 5).
|
Duration |
Total Iron Binding Capacity (µg/dL) (mean ± SD) |
||
|
Health Drink Mix Group |
Control Group |
P value |
|
|
Baseline |
399.05±65.82 |
381.41±73.19 |
0.33 |
|
Day 30 |
407.53±65.90 |
385.29±70.75 |
|
|
Day 60 |
390.59±66.95 |
384.71±73.07 |
|
|
Day 90 |
385.36±67.29 |
384.74±73.07 |
|
|
Mean diff |
-8.48±3.52 |
-3.88±6.59 |
0.001 |
|
(Baseline – Day 30) |
|||
|
Mean diff |
8.46 ±7.53 |
-3.30±4.29 |
<0.001 |
|
(Baseline – Day 60) |
|||
|
Mean diff |
13.69±8.28 |
-3.33±4.30 |
<0.001 |
|
(Baseline – Day 90) |
|||
Data analyzed by student t test. Significant at p<0.05.
Table 5: Changes in mean total iron binding protein (TIBC) levels between groups
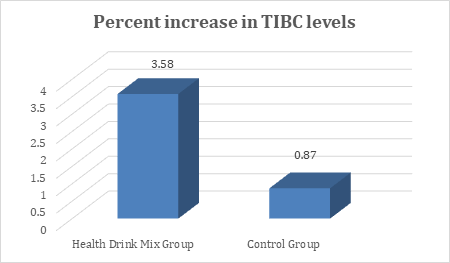
Figure 5: Changes in percent increase in mean total iron binding protein levels between groups
Changes in mean hematological parameters
In the present study, the hematological parameters like hematocrit, MCV, MCH and MCHC were comparable. There was marginal increase in levels of hematocrit, MCV, MCH and MCHC in both groups (table 6).
|
Hematocrit (%) (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
32.82±4.13 |
32.41±3.89 |
0.6912 |
|
Day 30 |
32.92±4.18 |
32.47±3.91 |
0.3927 |
|
Day 60 |
32.69±4.32 |
32.86±3.59 |
0.2201 |
|
Day 90 |
32.71±4.31 |
32.87±3.59 |
0.2271 |
|
MCV fL (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
76.18±11.17 |
77.40±9.94 |
0.6566 |
|
Day 30 |
76.32±11.29 |
77.50±10.00 |
0.7024 |
|
Day 60 |
76.50±11.31 |
75.66±13.83 |
0.3114 |
|
Day 90 |
76.51±11.31 |
75.67±13.79 |
0.3092 |
|
MCH pg (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
24.60±4.09 |
25.35±4.47 |
0.4992 |
|
Day 30 |
24.63±4.19 |
25.12±3.80 |
0.4901 |
|
Day 60 |
24.86±4.26 |
25.17±3.79 |
0.2196 |
|
Day 90 |
24.88±4.26 |
25.07±3.83 |
0.1424 |
|
MCHC g/dL (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
32.27±1.76 |
32.13±2.04 |
0.7714 |
|
Day 30 |
32.26±1.76 |
32.10±2.02 |
0.5346 |
|
Day 60 |
32.36±1.77 |
32.15±2.04 |
0.0361 |
|
Day 90 |
32.38±1.77 |
32.16±2.04 |
0.0292 |
Data analyzed by student t test. Significant at p<0.05.
Table 6: Changes in mean hematological parameters
Changes in clinical symptoms score between groups
In present study, subjects with both groups presented mild to moderate symptoms (score more than 5 on 0-10 VAS scale) like weakness, pale and yellow skin, dysmenorrhea, lightheadedness etc. which are related to anemia. After treatment there was significant reduction in symptoms till day 90. If compared between groups the Health Drink Mix group demonstrated significant reduction in symptoms compared to control group (table 7).
|
Weakness Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
7.03±0.85 |
6.93±0.78 |
0.6377 |
|
Day 90 |
*3.93±0.83 |
5.90±0.80 |
|
|
Mean diff |
3.10±3.40 |
1.03±0.41 |
<0.001 |
|
(Baseline – Day 90) |
|||
|
Pale and Yellow Skin Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
6.03±0.81 |
5.80±0.81 |
0.2673 |
|
Day 90 |
*1.90±0.80 |
4.77±0.82 |
|
|
Mean diff |
4.13±0.51 |
1.03±0.18 |
<0.001 |
|
(Baseline – Day 90) |
|||
|
Irregular Heartbeats Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
4.93±0.74 |
5.10±0.80 |
0.4065 |
|
Day 90 |
*1.87±0.73 |
3.07±0.78 |
|
|
Mean diff |
3.06±0.37 |
2.03±0.18 |
0.6563 |
|
(Baseline – Day 90) |
|||
|
Dysmenorrhea Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
8.03±0.76 |
7.93±0.78 |
0.6191 |
|
Day 90 |
*5.07±0.78 |
6.50±0.79 |
|
|
Mean diff |
2.96±0.41 |
1.43± 0.64 |
0.016 |
|
(Baseline – Day 90) |
|||
|
Dizziness Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
5.03±0.76 |
5.03±0.81 |
1 |
|
Day 90 |
*1.83±0.75 |
4.07±0.78 |
|
|
Mean diff |
3.20±0.61 |
0.96±0.41 |
0.001 |
|
(Baseline – Day 90) |
|||
|
Lightheadedness Score (Mean ± SD) |
|||
|
Duration |
Test |
Control |
P value |
|
Baseline |
5.13±0.78 |
4.97±0.76 |
0.4056 |
|
Day 90 |
*2.03±0.76 |
3.97±0.81 |
|
|
Mean diff |
3.10±0.45 |
1.00±0.26 |
0.031 |
|
(Baseline – Day 90) |
|||
|
Cold Hands and Feet Score (Mean ± SD) |
|||
|
Duration |
Test |
Control |
P value |
|
Baseline |
5.10±0.80 |
4.93±0.78 |
0.4195 |
|
Day 90 |
*2.00±0.74 |
2.95±0.76 |
|
|
Mean diff |
3.10±0.48 |
1.98±0.32 |
0.012 |
|
(Baseline – Day 90) |
|||
Data analyzed by student t test. Significant at p<0.05. *denotes significant within group
Table 7: Changes in clinical symptoms score between groups
Changes in quality of life score between groups
At baseline, the quality of life score of subjects with both groups were comparable with each other. In the present study, there was significant improvement in quality of life score in Health Drink Mix treated group than control group after 90 days of treatment (table 8).
|
SF-36 Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
71.23±3.65 |
70.97±2.28 |
0.7353 |
|
Day 90 |
101.50±2.85 |
101.17±2.42 |
|
|
Mean diff |
-30.27±3.54 |
-30.20±2.71 |
<0.001 |
|
(Baseline – Day 90) |
|||
Data analyzed by student t test. Significant at p<0.05.
Table 8: Changes in quality of life score between groups
Changes in fatigue severity score between groups
At baseline, the fatigue severity score was comparable between groups. At day 90, there was significant reduction in fatigue severity score in Health Drink Mix treated group than control (table 9, figure 6).
|
FSS Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
58.63±1.67 |
58.57±1.14 |
0.8571 |
|
Day 90 |
26.43±1.85 |
40.57±1.07 |
|
|
Mean diff |
32.20±2.43 |
28.00±1.78 |
<0.001 |
|
(Baseline – Day 90) |
|||
Data analyzed by student t test. Significant at p<0.05.
Table 9: Changes in fatigue severity score between groups
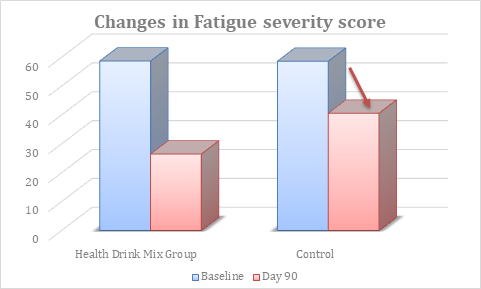
Figure 6: Changes in fatigue severity score between groups
Changes in energy audit score between groups
There was comparable score of high energy events at baseline in all subjects of both groups. After treatment, at day 90 there was significant increase in high energy event score in Health Drink Mix group compared to control group (table 10). There was 133% increase in high energy events in Health Drink Mix treatment group compared to 51% increase in control group.
|
High energy event Score (Mean ± SD) |
|||
|
Duration |
Health Drink Mix Group |
Control |
P value |
|
Baseline |
3.43±1.14 |
3.27±1.08 |
0.5625 |
|
Day 90 |
8.00±0.74 |
4.97±0.81 |
|
|
Mean diff (Baseline – Day 90) |
-4.57±1.25 |
-1.70±0.65 |
<0.001 |
Data analyzed by student t test. Significant at p<0.05.
Table 10: Changes in energy audit score between groups
Changes in CLBT Score between groups
At baseline the subjects from both of the groups were experiencing dark under eye circles, less skin luminosity and dullness of skin. After treatment, at day 90 there was significant reduction in dark circle score and significant increase in skin luminosity and brightness score in Health Drink Mix treated group than control (table 11).
|
(Mean ± SD) Dark Circles score |
|||
|
Duration |
Test |
Control |
P value |
|
Baseline |
7.47±0.51 |
7.43±0.50 |
0.7994 |
|
Day 90 |
4.40±0.56 |
2.50±0.51 |
|
|
Mean diff (Baseline – Day 90) |
3.07±0.69 |
4.93±0.87 |
<0.001 |
|
(Mean ± SD) Luminosity score |
|||
|
Duration |
Test |
Control |
P value |
|
Baseline |
2.57±0.50 |
2.47±0.51 |
0.4468 |
|
Day 90 |
7.50±0.51 |
5.57±0.50 |
|
|
Mean diff (Baseline – Day 90) |
-4.93±0.78 |
-3.10±0.76 |
<0.001 |
|
(Mean ± SD) Brightness score |
|||
|
Duration |
Test |
Control |
P value |
|
Baseline |
3.53±0.51 |
3.47±0.51 |
0.6127 |
|
Day 90 |
7.40±0.50 |
5.43±0.50 |
|
|
Mean diff (Baseline – Day 90) |
-3.87±0.86 |
-1.9±0.61 |
<0.001 |
Data analyzed by student t test. Significant at p<0.05.
Table 11: Changes in CLBT Score between groups
3. Discussion
In the present study, we compared the impact of Multigrain Instant Health Drink Mix versus counselling and education over nutritional choices in clinical management of anemia in women. The subjects participated in the study were anemic with the Hb levels less than 12 g/dl. All baseline hematological and symptomatological status of the participants in both groups were comparable. This avoids the bias and promised data integrity and robustness. After treatment with Multigrain Instant Health Drink Mix, in 30 days the mean Hb levels raised by 11.5% continued to increase till day 90 and achieved 20.59% increase in Hb levels at the end of the study. The serum iron, ferritin and TIBC levels were also significantly increased after treatment with Multigrain Instant Health Drink Mix. There was improvement in quality of life, high energy events during the day after treatment of Multigrain Instant Health Drink Mix. There was significant reduction in anemia related symptoms eventually from baseline to day 90 after treatment of Multigrain Instant Health Drink Mix. The dark under eye circle score was reduced and improvement in skin luminosity and brightness was observed. Overall, as an intervention Multigrain Instant Health Drink Mix was able to be effective on different levels in therapeutics of anemia- 1) showed improvement in gold standard parameter for treatment of anemia like Hb, serum iron, TIBC etc. 2) demonstrated improvement in symptomatology providing enhanced quality of life to subjects with improved daily energy status 3) there was less fatigue experienced by subjects after treatment with Multigrain Instant Health Drink Mix. Together with improved skin luminosity, brightness and reduced under eye dark circles this can provide opportunity to women to be more productive and confident persona. From the safety perspective, there were no adverse events related to Multigrain Instant Health Drink Mix treatment indicated safety of consuming Multigrain Instant Health Drink Mix for longer duration like 3 months. Oral iron is the gold standard to treat mild-to moderate IDA (Iron Deficiency Anemia) [4]. The major problem with oral iron therapy in its classic ferrous form is poor tolerability and its high adverse reaction rate, which can be as high as 40% in some cases [5]. The most common complaints are nausea, abdominal pain, diarrhea, and constipation [6]. It is also associated with non-sustainable efficacy. Hence, there is a crucial need to develop novel approaches and come up with nutrition based safe and efficacious product which can be better tolerated and assimilated in lifestyle to provide multiple benefits to patient with anemia. Nutrition can provide effective and safe or tolerable iron supplements, hence the present study was planned to evaluate the efficacy and safety of Multigrain Instant Health Drink Mix formulation, in the treatment of IDA. A sufficiently low hemoglobin by definition makes the diagnosis of anemia, along with low hematocrit value. Further studies will be undertaken to determine the anemia's cause. In case of iron deficiency anemia- ferritin, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin are on lower side and total iron-binding capacity, transferrin, red blood cell distribution width are on higher side. The clinical management is intended towards normalizing the imbalance [12,13]. In present study, it is observed that there was significant increase in Hb, serum iron, ferritin levels compered to control and baseline value. The mean corpuscular volume, mean corpuscular hemoglobin were also increased after the treatment increased compared to baseline but not statistically significant when compared to control. The improvement in these parameters reinforces the efficacy of the Health Drink Mix as an iron supplementation. Our data suggested that there was reduction in the total iron-binding capacity from baseline to day 90. Collectively it is well evident fact that the treatment with Multigrain Instant Health Drink Mix has modulated the hematological parameters indicating better therapeutic advantages in the treatment of anemia. As per the previous researches it is demonstrated that Hb production had a relationship with the level of serum iron. In IDA with oral iron supplementation, patients could achieve serum iron values between 70 μg/100 ml and 150 μg/100 ml. With intravenous iron dextran, the iron supply increased the serum iron to values greater than 200 μg/100 ml. In our study, the serum iron levels were low at baseline, and improved significantly after the therapy with the health drink mix, to the levels similar to the oral iron supplementation [7]. Subjective assessment based on the anemia symptomatology showed statistically significant improvement in grades of symptoms in the Health Drink Mix significantly more than control groups. The common symptoms of anemia like pale and yellow skin, irregular heartbeats, dysmenorrhea, dizziness, lightheadedness, cold hands and feet were resolved with treatment of health drink mix. 36-Item Short Form Health Survey is the most widely used generic well-standardized measure of HRQOL (Health related quality of life) used in healthy and various diseased populations. It consists of 36 questions measuring eight health domains that include physical and emotional aspects. These eight domains are combined into two component summary measures: the physical component and the mental component Summary scores. Previous studies of patients with anemia have documented that almost all of the SF-36 domains are impacted, with the greatest perturbation involving those related to physical activity and physical function. However, as noted below, even those domains focusing on role limitations due to emotional health and overall general health perception are affected. In the present study, the subjects treated with Multigrain Instant Health Drink Mix presented improvement in physical activity and performance domain together with emotional component score and overall wellbeing and presented significant improvement in total SF 36 score. Subjects provided additional feedback of experiencing more bone strength and stamina [8,9]. Common symptoms of IDA include fatigue and exercise-associated dyspnea, poor mental performance and cold intolerance which further accentuate fatigue. Research involving anemic patients has found an increase in hemoglobin is associated with an improvement in fatigue, in turn is associated with improvements in health related quality of life (HRQL) [10]. There is also evidence that decreases in hemoglobin are related to increases in fatigue duration. Fatigue is a burdensome symptom in iron deficiency anemia (IDA) [11]. To capture the severity and impact of fatigue appropriately it must be measured using validated scales [12]. In the present study, the fatigue severity score (FSS) was significantly reduced suggesting of reduced fatigue in Multigrain Instant Health Drink Mix treated group. Energy audit questionnaire is a tool used in research to understand subject perception to energy levesl throughout day. As the impact of fatigue and anemia, in the present study, there were very less number of events recorded where subject reported high energy during the day but eventually after 12 weeks of treatment with Multigrain Instant Health Drink Mix the frequency and score of high energy events reported by subjects were significantly increased. There are several symptoms related to skin health due to anemia in women. It is researched fact that the complexion, color tone is impacted by IDA together with indication such as hyperpigmentation and dullness of skin [14]. This in turn adds up to the emotional connection leading to diminishing overall wellbeing and self-esteem for women. It is important aspect of management of anemia to address the skin related symptoms in anemia. In the present study, it was observed that after treatment with Multigrain Instant Health Drink Mix there was improvement in skin luminosity, brightness and reduction in under eye dark circles. Prevalence of anemia among women between the ages of 15 and 49 is more than 40% in most Asian and African countries. Many factors cause IDA including, gut health, dietary iron deficiency, bioavailability, folic acid deficiency, Vitamin C, Vitamin A, and Vitamin B12 deficiency [15]. Three major approaches are followed to control IDA globally, which are supplementation with iron and folic acid tablets, fortification and natural food-based approaches. Despite the wide implementation of the first two approaches, IDA remains a serious malnutrition problem with an increasing trend globally. The third approach mainly focuses on dietary diversification and enrichment of diets with naturally iron-rich foods without the potential side effects of artificial additives [16]. In developing as well as developed countries, milled rice, wheat, and maize replaced the traditional nutritious crops. Refined foods are abundant in starch but lack nutrients, especially micronutrients such as iron (Fe) and zinc (Zn). Given that a major part (>80%) of the diet comprises low iron staple food, achieving sufficient intake of iron through the remaining 20% of the diet is impractical [17]. Therefore, it is important to diversify the staple food by including naturally iron-rich food crops such as millets. In addition, millets have a 2.3 to 4.0 times more dietary fiber (6.4 ± 0.6 to 11. 5 ± 0.6 g/100g) compared with refined rice and refined wheat, which acts as food for beneficial gut microbiome that improves abundance and alters the gut composition in a beneficial way [18]. Millets have added advantages as they are recognized as a smart food, i.e., not only good for you since it is nutritious and healthy, but also good for the planet because it is environmentally sustainable and good for farmers since it is resilient and climate-smart [19,20]. The results observed in the present study are outcomes of the composition and quality of Multigrain Instant Health Drink Mix. It’s a unique composition iron Fortified Women's Health Drink which delivers 100% RDA of Iron, Vitamin C, Vitamin B9 and B12 together with all the nutritional goodness of millets. Vitamin C helps increase iron absorption, along with vitamin B9 and B12 improves oxygen supply and red cells. Iron increases the hemoglobin production. In the present study, it is observed that Multigrain Instant Health Drink Mix is safe in women with anemia presenting no clinical and biochemical signs of any abnormal changes including no adverse events which are related to study intervention.
4. Conclusion
It can be concluded from the present study that Multigrain Instant Health Drink Mix is safe and efficacious in treatment of anemia in women. It can offer 360 degree improvement in anemia. It ensures improvement in Hb, Ferritin, Iron levels along with reduction in TIBC. Treatment with Multigrain Instant Health Drink Mix ascertained the fact that it can provide a wholesome improvement in quality of life, symptoms alleviation and improved skin tone and luminosity. These results can provide a firsthand evidence to the fact that there are no adverse events related to study intervention alike the oral iron supplementation in the treatment of anemia. It indicates that the iron and other micro-nutrition delivered through the Multigrain Instant Health Drink Mix is more assimilated and provided the sustainable results for anemia. The present research ensures consumers of this product to get the effectiveness to treat and manage the anemia and related complaints with no side effects related to iron supplementation.
Acknowledgments
The authors would like to acknowledge the research team and the back-office team involved in the research work. We would like to acknowledge the support from all study site staff, investigators and back office team.
Declaration of conflict of interests
None
Funding
The study medication was provided by Southern Health Foods Pvt Ltd, Chennai along with the expenses for laboratory testing for study participants.
References
- WHO |Anemia. WHO (2019).
- Balarajan Y, Ramakrishnan U, Özaltin E, et al. Anaemia in low-income and middle-income countries. Lancet 378 (2011): 2123-2135.
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One 10 (2015): e0117383.
- Breymann C, Dudenhausen JW. Iron deficiency in women. Handbook of Famine, Starvation, and Nutrient Deprivation. Springer International Publishing, Cham, Switzerland. 1 (2017): 1-14.
- Bhavi SB, Jaju PB. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy. A randomized controlled trial. BMC Pregnancy Childbirth. 17 (2017): 137.
- Van Wyck DB, Roppolo M, Martinez CO, et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int 68 (2005): 2846-2856.
- Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 4 (2011): 177-184.
- Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Curr Med Res Opin 23 (2007): 2997-3008.
- Thein M, Ershler WB, Artz AS, et al. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine 88 (2009): 107-114.
- Acaster S, Dickerhoof R, DeBusk K, et al. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual Life Outcomes 60 (2015).
- Cella D, Kallich J, McDermott A, et al. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol 15 (2004): 979-986.
- Lind M, Vernon C, Cruickshank D, et al. The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br J Cancer 86 (2002): 1243-1249.
- Bope ET, Kellerman RD. Conn's Current Therapy. Philadelphia: Elsevier 12 (2018): 403-405.
- Thomason RW, Almiski MS. Evidence that stainable bone marrow iron following parenteral iron therapy does not correlate with serum iron studies and may not represent readily available storage iron". American Journal of Clinical Pathology 131 (2009): 580-585.
- Goodarzi A, Behrangi E, Sadeghzadeh-Bazargan A, et al. The association between melasma and iron profile: a case-control study. Russian Open Medical Journal 9 (2020).
- Anitha S, Kane-Potaka J, Botha R, et al. Millets can have a major impact on improving iron status, hemoglobin level, and in reducing iron deficiency anemia–a systematic review and meta-analysis. Frontiers in Nutrition 12 (2021): 712-718.
- Anitha S, Kane-Potaka J, Tsusaka TW, et al. Acceptance and impact of millet-based mid-day meal on the nutritional status of adolescent school going children in a peri urban region of Karnataka state in India. Nutrients 11 (2019): 1-16.
- Vetriventhan M, Azevedo VCR, Upadhyaya HD. Genetic and genomic resources, and breeding for accelerating improvement of small millets: current status and future interventions. Nucl. 63 (2020): 217–239.
- Longvah T, Ananthan R, Bhaskarachary K, et al. Indian Food Composition Table. Hyderabad: National Institute of Nutrition 11(2017): 578.
- Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr. 106 (2017): 1688-1693.
