A Patient with Hypercalcemia Who Turned Out to Have Multiple Endocrine Disorders
Article Information
Zaina Alamer*, Khaled Baagar, Zaina Rohani, Mahmoud Zirie
Department of Endocrine, Hamad Medical Corporation, Doha, Qatar
*Corresponding Author: Zaina Alamer, Department of Endocrine, Hamad Medical Corporation, Doha, Qatar
Received: 18 November 2021; Accepted: 26 November 2021; Published: 06 December 2021
Citation: Alamer Z, Baagar K, Rohani Z, Zirie M. A Patient with Hypercalcemia Who Turned Out to Have Multiple Endocrine Disorders. Archives of Clinical and Medical Case Reports 5 (2021): 927-940.
Share at FacebookAbstract
Multiple endocrine neoplasia type 1 (MEN1) is rare and characterized by 3 main endocrine tumors (parathyroid, enteropancreatic, and pituitary). We report a patient with MEN1 and present a comprehensive literature review. A 30- year-old man presented with abdominal pain. He was vitally stable, dehydrated with coarse facial features, nodular goiter, and trunk neurofibroma-like lesions. Laboratory showed normal amylase and lipase, calcium 3.2 (2.20-2.55 mmol/L), phosphorous 0.67 (0.81-1.45 mmol/l), and PTH 400 (15-65 ng/L). He received intravenous saline, calcitonin, and zoledronate. During hospitalization, he developed hypoglycemia 1.6 mmol/L with C-peptide 5.48 (1.10-4.4 ng/mL), and insulin 22.8 (2.6-24.9 mcunit/mL). Sestamibi scan revealed parathyroid gland hyperplasia. MRI abdomen showed a pancreatic lesion suggestive of insulinoma. He had prolactin > 100000 (85-323 mIU/L), IGF-1 456 (96-228 ug/L), secondary hypogonadism and secondary hypothyroidism. Pituitary MRI showed giant macroadenoma. MEN1 was clinically diagnosed and confirmed by genetic testing. Thyroid nodules FNA showed follicular neoplasm. The multidisciplinary team recommended cabergoline, insulinoma surgery, and total thyroidectomy plus total parathyroidectomy with autoimplantation of the parathyroid. Histopathology confirmed a well-differentiated pancreatic neuroendocrine tumor, and a 9 mm Papillary Thyroid Cancer (PTC) (pT1aN0). Thereafter, He had no hypoglycemia; however, he developed hypoparathyroidism. We kept him on thyroxine, calcitriol, and calcium and monitored his response to cabergoline.
MEN1 can be diagnosed genetically, or clinically by having 2 of the main 3 tumors or 1 tumor in a first-degree relative of a MEN1 patient. Genetic testing is essential for index cases and unaffected at-risk relatives to decide further screening. The MEN1 individual lesions management is generally similar to sporadic cases; however, patient
Keywords
Autosomal dominant inherited disorder; Multiple endocrine neoplasia type 1
Autosomal dominant inherited disorder articles; Multiple endocrine neoplasia type 1 articles
Article Details
1. Introduction
Multiple Endocrine Neoplasia Type 1 (MEN1) is a rare autosomal dominant inherited disorder with an estimated prevalence of 0.22-0.25% [1- 4]. It is characterized by the presence of three main endocrine tumors [parathyroid adenomas, enteropancreatic neuroendocrine tumors (EP-NETs), and pituitary tumors] [1,5,6]. There are other tumors that can be associated with MEN1 including carcinoid (thymic, bronchial and gastric), adrenocortical tumors, central nervous system tumors (meningiomas, schwannomas, and ependymomas), and skin tumors (angiofibromas, collagenomas, and lipomas). On the other hand, Papillary Thyroid cancer (PTC) has been reported in MEN1 cases; However, a clear association has not been established. An inactivating mutation in the MEN1 tumor suppressor gene is implicated in the tumorigenesis of MEN1. Here we present a case of MEN1 and PTC, with a literature review of MEN1 genetics, diagnosis, management, and screening recommendations.
2. Case Report
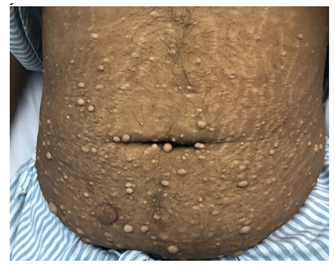
This is a 30-year-old male with a past medical history of hypercalcemia induced pancreatitis 5 months prior to this presentation; however, at that time, he asked for discharge against medical advice after 2 days of hospital admission. This time he presented to the emergency department with a history of nausea, vomiting, and abdominal pain. On physical examination, he looked dehydrated, otherwise vitally stable. He had coarse facial features, an enlarged tongue with wide-spaced teeth, and his hands and feet were large. He had a normal visual field by confrontation test. He had galactorrhea on breasts expression. He had Multinodular Goiter (MNG) with the largest palpable 2.5 cm nodule on the left side. Also, he had fleshy neurofibroma-like skin lesions mainly on the abdomen, neck, and axillae (Figure 1), with dark pigmentation of the axilla and groin, which had been there for 10 years.
His initial blood works were significant for corrected calcium: 3.23 mmol/L (2.20-2.55), potassium: 2.5 mmol/l (3.5-5.1), phosphorous: 0.67 mmol/l (0.81-1.45), magnesium: 0.62 mmol/L (0.66-1.07), amylase 27 U/L (13-53), Lipase 24 IU/L (13-60), vitamin D: 15 ng/ml (30-75), parathyroid hormone (PTH) 400 pg/ml (15.0-65.0) indicating primary hyperparathyroidism(PHPT). He received intravenous normal saline with potassium chloride, calcitonin, and zoledronic acid. While he was in the emergency department, he developed an episode of hypoglycemia with plasma glucose 1.6 mmol, C-peptide 5.48 ng/ml (1.10-4.4), and insulin 22.8 mcunit/ml (2.6-24.9) indicating endogenous excess insulin production.
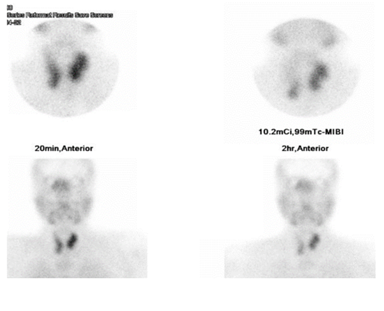
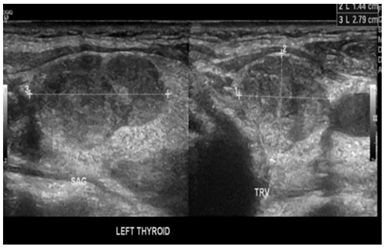
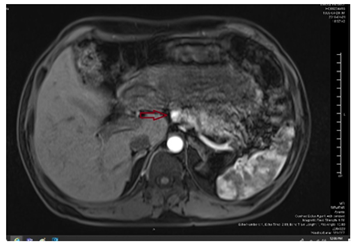
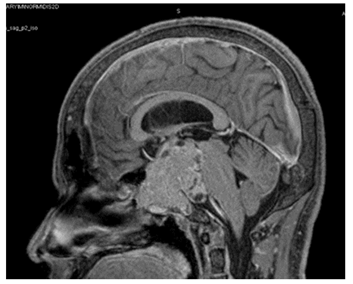
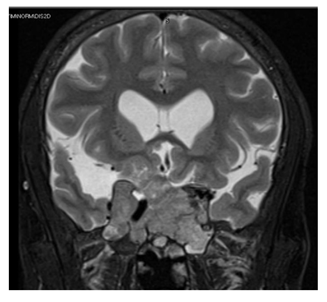
Our working diagnosis was MEN-1. Further extensive work-up revealed prolactin > 100000 mIU/L (85-323), insulin-like growth factor -1 (IGF-1) 456 ug/L (96-228), secondary hypogonadism; testosterone : 1.27 nmol/l (10.4-37.44), FSH : 1.8 Iu/l (1.5-12.4), and LH : 1.5 Iu/l (1.7-8.6), central hypothyroidism with TSH : 0.58 mIu/L (0.30-4.2), and FT4 : 10.6 pmol/l(11.6-21.9), and cortisol 239 nmol/L (133-537). A confirmatory growth hormone (GH) suppression test was diagnostic of acromegaly (GH at 0 minutes: 19.83 mcg/l, 60 minutes: 15.16 mcg/l, 90 minutes: 14.39 mcg/l, and 120 minutes: 14.49 mcg/l). Adrenal insufficiency was excluded by a short synacthen test. Sestamibi scan was suggestive of hyperplasia of the parathyroid glands (Figure 2). Ultrasound kidneys showed nephrocalcinosis. Ultrasound neck showed multiple hypoechoic thyroid nodules with the largest on the left side sized 1.8 X 1.4 X 2.7 cm (Figure 3). Thereafter, ultrasound-guided Fine Needle Aspiration (FNA) biopsy from the largest nodule revealed follicular neoplasm with hurthle cells. Magnetic Resonance Imaging (MRI) abdomen demonstrated an exophytic pancreatic lesion with features suggestive of insulinoma (figure 4). Also, it showed a left adrenal adenoma of 5 X 5 mm. Pituitary MRI showed giant pituitary macroadenoma extending to cavernous sinuses, posterior fossa, and sphenoid sinus. (Figure 5A and B).
At that point, MEN-1 syndrome was clinically confirmed with primary hyperparathyroidism, insulinoma, and functioning giant pituitary macroadenoma with excess prolactin and growth hormone production. Also, the patient had MNG.
- Insulinoma: surgical enucleation was done. Histopathology confirmed a well-differentiated neuroendocrine tumor grade 1. Thereafter, he had no hypoglycemic episodes.
- Primary hyperparathyroidism (PHPT): cinacalcet 60 mg twice daily was required to control the hypercalcemia until the patient underwent total parathyroidectomy with auto-implantation of parathyroid in the forearm, after surgery, the patient developed hypocalcemia which required treatment with calcitriol 0.25 mcg daily and calcium carbonate 1250 mg three times daily.
- Total thyroidectomy at the same surgery for parathyroidectomy: a 0.9 mm papillary thyroid cancer (PTC), follicular variant, was diagnosed without lymphovascular invasion (pT1aN0) and the patient continued on levothyroxine 100 mcg/day as a replacement.
- Genetic testing was done for the patient and confirmed the presence of MEN1-associated mutation.
3. Discussion
MEN1 is a rare autosomal dominant inherited disorder. It is characterized by the presence of three main endocrine tumors (parathyroid adenomas, EP-NETs, and pituitary tumors) [1,4,6], in addition, patients may have other less common tumors. These tumors can be diagnosed as early as 5 years of age and up to 81 years [1,2]. Our patient presented at the age of 30 years with the 3 main tumors.
3.1. Genetic background of MEN1
MEN1 is caused by a mutation in the MEN1 tumor suppressor gene on chromosome 11q13 [1,6,7] which encodes the protein menin. It is responsible for genome stability and regulation of cell division and proliferation [1,6,8]. Until now there is no direct phenotypic and genotypic correlation [1,3,6,9]. However, there is still an important clinical implication for detecting the germline mutation, as first degree relatives of MEN1 patients have a 50 % chance of developing the disease [1,2,6,10]. Mutation detection allows early diagnosis of the disease in the pre-symptomatic stage with timely intervention, reducing morbidity and mortality [1,2,6,9,11]. Also, it will spare the negatively tested relatives from the heavy burden of tumors screening [1,10]. Genetic counseling should be offered to all individuals undergoing testing [1,2]. A sporadic nonfamilial form of MEN1 has been reported but it is rare; 10% of MEN1 cases [1,6].
MEN1 patients have decreased life expectancy, with a mean age of death of 55-60 years [1,4,6,10]. The natural course of the disease has changed with the emergence of new treatments for hormonal hypersecretion, making the malignant EP-NETs are the most common cause of death followed by thymic carcinoid [1,4,6,9,11,12]. Data has shown that screening can improve prognosis by early tumor detection in asymptomatic individuals [1,2,6,9,11].
3.2. Diagnostic criteria of MEN1
Diagnosis of MEN1 can be either clinical; by detecting two out of the three main tumors or one main tumor in a first-degree relative of a confirmed MEN1 patient, or genetic by detecting the germline mutation in an individual who has no biochemical or radiological abnormalities [1,6]. our patient clinically had the three main tumors, and genetic testing also confirmed the diagnosis.
Genetic testing should be offered to index cases and all their first-degree relatives as early as possible starting from the age of 5 years [1,6,8]. Also, it may be offered to any patient with atypical MEN1 presentation such as having two non-classical tumors (e.g. adrenal and parathyroid adenoma) or a suspicious single tumor type such as PHPT diagnosed before the age of 30 years, multiglandular hyperparathyroidism, or multiple EP-NETs at any age [1,6].
3.3. Neoplasms associated with MEN1
3.3.1. Parathyroid tumors: Parathyroid tumors are the earliest and most frequent manifestation occurring in 90-95% of cases [1,8,13,6]. The mean age of onset is 20-25 years with an equal male to female ratio as opposed to sporadic primary hyperparathyroidism (SPHPT), which has an average age of onset of 55 years and is more common in females [1,6,14,13].
Our patient presented at the age of 30 years with hypercalcemia. Although the biochemical picture is usually milder in MEN1-associated PHPT; lower calcium and lower PTH levels, MEN1-related PHPT patients show earlier and more severe bone involvement as well as more frequent renal complications than SPHPT [1,6,14]; our patient had nephrocalcinosis at presentation. Severe hypercalcemia or parathyroid carcinoma is rare [1,6]. Multiple glands are usually involved in MEN1 PHPT with either adenoma or hyperplasia, which makes imaging with Ultrasound and sestamibi scan of limited benefit [1,5]. Our patient’s sestamibi scan showed multiple glands involvement. The mainstay of treatment is surgery and the indications for surgery are similar to SPHPT [1]. The only difference is for asymptomatic patients with SPHPT, age less than 50 years is an indication for surgery; however, in MEN-1 PHPT the age alone is not sufficient to recommend surgery [4]. Until now the timing and the extent of surgery are still debatable [1,4,5]. Thakker et al. recommended bilateral neck exploration in all patients, and suggested subtotal parathyroidectomy as the procedure of choice, as it has a similar rate of recurrence to total parathyroidectomy on the long run with lower rates of hypoparathyroidism [1,15]. Total parathyroidectomy and autoimplantation may be considered in selected patients with an extensive disease [1]. A recent report by montegrento et al proposed that less than subtotal parathyroidectomy may be considered in selected young patients with a mild, lateralized disease on preoperative imaging [15]; however, further studies are warranted in this area. The risk of recurrence of hypercalcemia post parathyroidectomy is higher in MEN1-PHPT reaching up to 40-60% post subtotal parathyroidectomy within 10-12 years compared to 4-16% in SPHPT [1,2,15]. Localization studies before re-operation may be useful for recurrent or persistent disease [2]. Patients who fail or unfit for surgery, medical treatment with cinacalcet is an option for treating their hypercalcemia [1,5]. Our patient underwent total parathyroidectomy with auto-implantation in the forearm which was complicated by hypocalcemia that was managed successfully with calcitriol and calcium carbonate supplements.
3.3.2. Enteropancreatic neuro-endocrine tumors (ep-nets): EP-NETs are the second most common tumors in MEN1, ranging from 40 to 70% in different reports [1,9,11], and they can be functioning or non- functioning. Among the functioning EP-NETs, gastrinoma is the most common (40%), followed by insulinoma 10-30% [1,6], which was the case in our patient who had an insulinoma in the pancreas as proved on histopathology. Other functioning EP-NETs (e.g. glucagonoma, VIPoma) are very rare (< 1%) [1]. The clinical presentation of functioning EP-NETs is similar to sporadic cases; however, they have an earlier age of onset; in the 4th decade of life [1,5], and are frequently small, multiple and deeply located compared to sporadic EP-NETs [1,5,9,11]. Also, although they are usually slowly growing, they can metastasis earlier than sporadic cases [1,5]. Two functioning EP-NETs can co-exist in 10% of the cases [5]. Surgical resection is the recommended treatment; however, it is challenging and sometimes not feasible because of the above-mentioned characteristics [1,2,5]. Preoperative localization is recommended as it improves the success rate of surgical treatment [1]. For inoperable tumors, the treatment is similar to sporadic cases [1]. Our patient was diagnosed with endogenous hyperinsulinemic hypoglycemia, and a pancreatic tumor was identified by MRI. Fortunately, he underwent a curative surgical removal of the culprit lesion.
Among the non- functioning EP-NETs, pancreatic NETs are the commonest reaching 55 % [1,5,11]. They are slowly growing, multiple, and present at a younger age than the sporadic cases, making their diagnosis and treatment challenging [1,11]. Additionally, they carry the worst prognosis due to their malignant potential and the ability to metastasize despite their indolent course [1,11]. Therefore, they are the leading cause of mortality in patients with MEN1 [4,5,9,11], and it is essential to screen and identify them as early as possible [1,11]. They were found to secrete hormones such as glucagon, chromogranin, and pancreatic polypeptide without any symptoms [1]. However, the recent reports recommend against routine biochemical testing as it is of low diagnostic accuracy [5,11]. On the other hand, imaging studies are currently the cornerstone for the surveillance of non- functioning pancreatic NETs [1,6,11,5]. Consistent data has shown that Endoscopic Ultrasound (EUS) is the most sensitive as it can detect small tumors sized down to 2 mm [1,5,11]; however, its sensitivity decreases in the pancreatic tail [11]. On the other hand, MRI is more convenient as it is non-invasive, with homogenous sensitivity throughout the pancreas, yet its overall sensitivity is less than the EUS [11]. The maximum diagnostic sensitivity can be achieved by combining both EUS and MRI alternatively as recommended by trijeneto et al. [11].
Another imaging modality that can be used for follow up of lesions sized more than 1 cm is Gallium DOTA- PET CT as it can identify metastasis early on [11]. However, it shouldn’t be used for screening as it exposes the patient to a high cumulative dose of ionizing radiation especially with the lifelong nature of the disease and starting screening at a young age [1,11]. Until now the age to start screening is controversial and the proposed age in previous reports is between 10 to 16 years [1,11]. Another controversial aspect is the treatment, the recommended treatment is surgical, but the cut-off size when to operate is still debatable given the multiple nature of the tumors and the complications associated with extensive surgery [1,5]. Thakker, R. V et al recommended surgery if the tumor exceeds 1 cm in diameter or if it shows significant growth, defined as doubling within 3-6 months [1]. On the other hand, cohort studies have shown that a conservative approach up to 2 cm may be safe and had no higher chance of metastasis [5,16]. This area deserves more attention as it is the leading cause of mortality and further studies are needed to improve the outcome.
3.3.3. Pituitary tumors: Pituitary tumors are the third most common tumors in MEN1 ranging from 15% to 50% in different studies [1,2,6], and they are rarely the first manifestation of the disease [5]. The average age of onset is in the fourth decade of life [2], and women are more affected than men (65% versus 35%) [1,9]. Macroadenomas are more common in MEN-1-related pituitary adenomas compared to sporadic pituitary tumors (85% vs 42%) [1,2]. They are more often to be functioning rather than non- functioning [1,2]. Functioning adenomas are most commonly prolactinomas (60%), followed by GH-secreting (25%), and adrenocorticotropic hormone (ACTH)-secreting adenomas (< 5%) [1,2]. Multihormonal secretion is also common, around 39% of the cases, and the most common combination is prolactin and GH co-secretion [2].
Our patient had an aggressive pituitary giant macroadenoma, co-secreting prolactin and GH, and he was started on medical treatment with cabergoline. MEN1 pituitary adenomas have a similar presentation to the sporadic ones, depending on the produced hormone(s); however, they tend to be larger in size, more aggressive in behavior, more resistant to treatment, and more likely multihormonal [1,2,3]. They have no increased risk of being malignant [3], and the recommended treatment is similar to patients without MEN1 [1]. In contrast to previous reports, de Laat et al proposed that pituitary tumors associated with MEN-1 were less aggressive than previously suggested and prolactinomas responded well to medical therapy [17]. Also, they reported that microadenomas in MEN1 patients that are asymptomatic and detected by screening showed an indolent course with a limited growth which was not clinically significant after many years, suggesting that less frequent MRI surveillance may be sufficient rather than what was previously recommended [1, 17]. However, more studies are needed to establish clear recommendations regarding this issue.
3.3.4. Adrenal tumors: Adrenal lesions can be associated with MEN1, affecting between 20% and 73% in different reports [1,5,6,8]. However, they had never been reported as the earliest manifestation of the disease [9]. The majority are hyperplastic and non- functioning [1,5]. Functioning adrenal lesions constitute < 10 %, and mainly associated with hyperaldosteronism or Cushing’s syndrome, while pheochromocytoma is rare (<1%) [1,5,6]. Adrenal Cell Carcinoma (ACC) was also reported and it was found to be more prevalent in MEN1 patients than sporadic cases with adrenal lesions [1,5,6]. Biochemical screening is similar to sporadic adrenal incidentalomas and is indicated if symptomatic or the size is more than 1 cm [1,5,6]. Surgical resection is indicated if the size is more than 4 cm, the tumor has grown significantly within 6 months, suspicious or atypical features are present, or if it is functioning [1,5,6], otherwise it is recommended to follow the lesions with CT or MRI [1,6]. Our patient had a non- functioning 0.5 cm adrenal lesion.
3.3.5. Thymic, bronchopulmonary, and gastric carcinoid: Carcinoid tumors have been reported in patients with MEN1 [1], and they can be located in the bronchi, thymus, and gastrointestinal tract.
3.3.6. Thymic carcinoid: Thymic carcinoid prevalence in MEN1 reaches (2-8%), with male predominance [1,5,6]. They are usually asymptomatic until late and tend to have an aggressive course [1,5]. Currently, they are the second leading cause of MEN1-related mortality next to EP-NETs [1,4-6]. Consistent hormonal abnormality has not been found and biochemical screening is not recommended [1,6]. On the other hand, radiological surveillance is the recommended modality until now by either CT or MRI of the chest every 1-2 years [1,6]. Because of the aggressive nature of the thymic carcinoid, prophylactic cervical thymectomy has been advised at the time of parathyroidectomy [1,6]; however, surveillance by imaging should continue as recommended [1,6]. The optimal treatment is surgical resection, if possible [1,6]. If curative surgery is not feasible due to invasive or metastatic disease, chemotherapy and radiotherapy can be used similar to sporadic cases. In addition, somatostatin analogs such as octreotide or lanreotide have shown benefit in improving symptoms and tumor regression [1,6].
3.3.7. Bronchopulmonary carcinoid: bronchial carcinoid has shown a female predominance in MEN1 [1,6]. It has not shown increased mortality but rather a favorable prognosis [1,6]. Similar to what applies to thymic carcinoid with regards to biochemical testing and screening by imaging also applies to bronchopulmonary carcinoid [1,6]. The treatment of choice is surgery if it is feasible [1,6].
3.3.8. Gastric carcinoid: Gastric carcinoids are associated with MEN1 [1,6]. These tumors are usually small and multiple [1,6], and the suggested screening tool is a gastroscopic examination with a biopsy every 3 years in patients who have hypergastrinemia according to Thakker, R. V. et al [1]. However, more recent data has suggested instead to do the endoscopic examination annually [6]. No clear recommendation for treatment has been established but in small lesions up to 10 mm endoscopic surveillance may be considered [1], while in larger lesions endoscopic resection or local resection with partial or total gastrectomy may be needed [1]. Also, somatostatin analogs are associated with tumor regression [1].
3.3.9. Thyroid tumors: Thyroid tumors have been reported in MEN1 cases, but since their prevalence in the general population is high and the rarity of MEN1 syndrome, a clear association is not yet established [1,18]. A recent study about concomitant thyroid cancer in MEN1 patients suggested that clinically significant thyroid cancer is uncommon [18] and it is worth mentioning that all the cases had classical PTC; however, our patient had follicular variant PTC. Further studies are warranted to establish a clear association between thyroid tumors and MEN1 syndrome.
3.3.10. Breast cancer: Recent evidence has linked breast cancer to MEN1 [5,6], with an average age of onset of 48 years. However, there is no consensus on screening recommendations and it is not different from what is recommended for breast cancer screening in the general population [5]. More studies are required to assess the necessity for a different screening recommendation.
3.3.11. Skin lesions: Skin lesions such as angiofibromas (85%), collagenomas (70%), and lipomas (30%) were reported with MEN1 [1,6,9]. They often manifest before the neuroendocrine tumors [6,9]. They are usually not clinically significant, and treatment is usually warranted for cosmetic reasons [9].
4. Follow up of the Patients
Follow up of patients with MEN1 is one of the cornerstones in their management, as the tumors may develop up to 81 years of age [1,2], and their recurrence is more common than sporadic tumors [1]. Initially, follow up with clinical and biochemical testing should be done every 3-6 months and can be later extended to be every 6-12 months [1,2], while imaging should be performed every 12-36 months depending on the diagnosed tumors [1,2]. Another important aspect in the management is being under the care of a highly specialized MDT with experienced endocrinologists, oncologists, surgeons, radiologists, and histopathologists [1,2,5], in a facility that has a full diagnostic capacity [1].
5. Screening
Early pre-symptomatic diagnosis has been shown to improve morbidity and survival in patients with MEN1 [1,2,9,6]. Therefore, the individuals with positive MEN1 diagnosis, whether clinically or genetically, are candidates for a comprehensive biochemical and radiological screening [1,6] (Table 1). Hormonal screening for EP-NETs with chromogranin A, pancreatic polypeptide, and glucagon was recommended by Thaaker et al [1], while more recent data has shown it is of low diagnostic accuracy and has suggested against it [11]. Instead, annual imaging by MRI, EUS, or both used alternately was recommended [1,6,11]. The starting age for screening is controversial between 10-16 years of age [1,6,11]. Screening for the anterior pituitary tumors should be started at the age of 5 years using annual prolactin and IGF-1 levels, in combination with pituitary MRI every 3 years [1,6]. If any abnormality is found, then a full hypothalamic-pituitary hormonal testing should be performed [1,6,19].
|
Tumor |
Age to begin screening (years) |
Biochemical test (serum) annually |
Imaging test (time interval) |
|
Parathyroid |
8 |
Calcium, PTH |
None |
|
Gastrinoma |
20 |
Gastrin |
None |
|
Insulinoma |
5 |
Fasting glucose, insulin |
None |
|
Other EP-NETs |
16-Oct |
CgA, PPP, glucagon, VIP * |
MRI or EUS (annually) |
|
Anterior pituitary |
5 |
Prolactin, IGF-1 |
MRI (every 3 years) |
|
Adrenal |
<10 |
None unless signs or symptoms and/or tumor > 1cm |
MRI or CT (annually with pancreatic imaging) |
|
Thymic and bronchial carcinoid |
15 |
None |
CT or MRI (every 1-2 year) |
|
Gastric carcinoid |
NA |
None |
OGD & biopsy (every 3 years in case of hypergastrinemia) |
MEN1: multiple endocrine neoplasia type 1, PTH: parathyroid hormone, EP-NETs: enteropancreatic neuroendocrine tumors, CgA: chromogranin A, PPP: pancreatic polypeptide, VIP: vasoactive intestinal peptide, MRI: magnetic resonance imaging, EUS: endoscopic ultrasound, IGF-1: insulin-like growth factor 1, CT: computed tomography, OGD: oesophago-gastro-duodunescopy
* It was recommended by Thakker et all (2012), but more recent data did not recommend.
Table 1: Recommended biochemical and radiological surveillance for MEN1-associated tumors in patients with MEN1 and MEN1 mutation carriers (1).
6. Conclusion
MEN1 can be diagnosed genetically or clinically by having 2 of the main 3 tumors or 1 main tumor in a first-degree relative of a diagnosed individual. Genetic testing is essential for index cases and unaffected at-risk relatives to decide further screening. The MEN1 individual lesions management is almost similar to sporadic cases; however, patients require life-long surveillance. Pre-symptomatic screening is important to decrease morbidity and mortality, but it is extensive at the time being, and further studies are warranted to update the current recommendations as a lot of controversial aspects have come up in screening and management recommendations.
Our patient had the 3 main tumors and in addition, he had a non-functioning adrenal adenoma and a follicular variant PTC. He was thoroughly investigated and managed by an MDT according to the latest available recommendations. The PTC in MEN1 patients is usually clinically insignificant; however, the association between both needs further studies. National registries have an important role in improving knowledge about MEN1 and should be more implemented, all patients and their at-risk family members should be cared for by an experienced MDT in a fully equipped diagnostic facility with experience in treating MEN1 patients.
References
- Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). Journal of Clinical Endocrinology and Metabolism 97 (2012): 2990-3011.
- Gilis-januszewska A, Skalniak A, Hubalewska-dydejczyk A. Pituitary tumours in MEN1 syndrome -the new insight into the diagnosis and treatment. 70 (2019): 445-452.
- Vergès B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 (MEN1): Data from the France-Belgium MEN1 multicenter study. Journal of Clinical Endocrinology and Metabolism 87 (2002): 457-465.
- Ito T, Igarashi H, Uehara H, et al. (2013). Causes of death and prognostic factors in multiple endocrine neoplasia type 1: A prospective study:Comparison of 106 men1/zollinger-ellison syndrome patients with 1613 literature men1 patients with or without pancreatic endocrine tumors. Medicine (United States) 92 (2013): 135-181.
- Van Leeuwaarde RS, De Laat JM, Pieterman CRC, et al. The future: Medical advances in MEN1 therapeutic approaches and management strategies. Endocrine-Related Cancer 24 (2017): T179–T193.
- Kamilaris CDC, Stratakis CA. Multiple endocrine neoplasia type 1 (MEN1): An update and the significance of early genetic and clinical diagnosis. Frontiers in Endocrinology (2019).
- Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Human Molecular Genetics 6 (1997): 1169-1175.
- Thakker RV. Multiple endocrine neoplasia type 1 (MEN1). Best Practice and Research: Clinical Endocrinology and Metabolism 24 (2010): 355-370.
- Marini F, Giusti F, Brandi ML. Multiple endocrine neoplasia type 1: extensive analysis of a large database of Florentine patients. Orphanet J Rare Dis 8 (2018): 1-18.
- Manoharan J, Albers MB, Bartsch DK. The future: diagnostic and imaging advances in MEN1 therapeutic approaches and management strategies 24 (2017): 209-225.
- van Treijen MJC, van Beek DJ, van Leeuwaarde RS, et al. Diagnosing Nonfunctional Pancreatic NETs in MEN1: The Evidence Base. Journal of the Endocrine Society 2 (2018): 1067-1088.
- Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM: Monthly. Journal of the Association of Physicians 89 (1996): 653-669.
- Brandi ML, Gagel RF, Angeli A, et al. Consensus: Guidelines for diagnosis and therapy of MEN type 1 and type 2. Journal of Clinical Endocrinology and Metabolism 86 (2001): 5658-5671.
- Eller-Vainicher C, Chiodini I, Battista C, et al. Sporadic and MEN1-related primary hyperparathyroidism: Differences in clinical expression and severity. Journal of Bone and Mineral Research 24 (2009): 1404-1410
- De Menezes Montenegro FL, Brescia MDEG, Lourenço DM, et al. Could the less-than subtotal parathyroidectomy be an option for treating young patients with multiple endocrine neoplasia type 1-related hyperparathyroidism? Frontiers in Endocrinology 10 (2019): 1-10.
- Triponez F, Sadowski SM, Pattou F, et al. Long-term Follow-up of MEN1 Patients Who Do Not Have Initial Surgery for Small ≤2 cm Nonfunctioning Pancreatic Neuroendocrine Tumors, an AFCE and GTE Study. Annals of Surgery 268 (2018) 158-164.
- De Laat JM, Dekkers OM, Pieterman CRC, et al. Long-term natural course of pituitary tumors in patients with MEN1: Results from the Dutch MEN1 study group (DMSG). Journal of Clinical Endocrinology and Metabolism 100 (2015): 3288–3296.
- Hill KA, Yip L, Carty SE, et al. Concomitant Thyroid Cancer in Patients with Multiple Endocrine Neoplasia Type 1 Undergoing Surgery for Primary Hyperparathyroidism. Thyroid 29 (2019): 252-257.
- Goudet P, Dalac A, Le Bras M, et al. MEN1 disease occurring before 21 years old: A 160-patient cohort study from the Groupe d’étude des Tumeurs Endocrines. Journal of Clinical Endocrinology and Metabolism 100 (2015): 1568-1577.