A New Protection System to Avoid Major Bleeding at Puncture Site-Results from the First in Men Study
Article Information
Elias Noory*, Ulrich Beschorner, Thomas Zeller, Tanja Böhme
Department of Angiology and Cardiology II, University Heart Center Freiburg-Bad Krozingen, Bad Krozingen, Germany
*Corresponding author: Dr. Elias Noory, Department of Angiology and Cardiology II, University Heart Center Freiburg-Bad Krozingen, Südring 15, D-79189 Bad Krozingen, Germany
Received: 09 November 2020; Accepted: 17 November 2020; Published: 21 November 2020
Citation: Elias Noory, Ulrich Beschorner, Thomas Zeller, Tanja Böhme. A New Protection System to Avoid Major Bleeding at Puncture Site-Results from the First in Men Study. Cardiology and Cardiovascular Medicine 4 (2020): 717-728.
Share at FacebookAbstract
Background: The use of catheter-directed thrombolysis (CDT) is an effective option for the treatment of acute lower limb ischaemia, but has a high complication rate. Systemic bleeding and bleeding at the puncture site are particularly dreaded. Purpose: To evaluate the safety and effectiveness of the new vascular access protection system CaveoVasc® protection system in patients undergoing CDT.
Materials & Methods: The prospective, single-center single-arm CaveoVasc® study enrolled 20 patients with critical limb ischaemia (CLI) requiring treatment with CDT. Primary safety endpoint was the rate of all major bleedings (BARC > type 3) from the start to the end of the CDT procedure. Secondary safety endpoints were bleedings (BARC ≥ 1 and BARC ≥ 2) and adverse events at discharge and at 30 days. The primary performance endpoint was length of CDT. Secondary performance endpoints were the rate of subjects completing the CDT without early interruption due to access site complication, the rate of successful thrombolysis without bleeding complication and pain at access site.
Results: Twenty patients with CLI requiring treatment with CDT were enrolled. No major bleeding occurred at the access site. The CDT treatment continued for a mean of 16.5+/- 5.2 hours without interruption due to bleeding complications at the access site. In 5 cases minor bleeding (classified as BARC 1 and BARC 2) was documented but only two of these patients had a bleeding at the site of the puncture during the procedure. In 18 cases (94.7%) the CDT procedure was completed successfully and without early interruption. At the start of the CDT, the average pain score was 0.3 ± 1.1, 2.0 ± 2.3 after 6 hours, and 0.8 ± 2.0 at the end of the CDT procedure. Twelve adverse events had occurred by the 30 day-visit.
Conclusion: The stud
Keywords
Acute limb ischemia; Peripheral artery disease; Thrombectomy; Catheter directed thrombolysis; Protection device; Bleeding complications
Acute limb ischemia articles; Peripheral artery disease articles; Thrombectomy articles; Catheter directed thrombolysis articles; Protection device articles; Bleeding complications articles
Article Details
Abbreviations:
ALI: Acute Limb Ischemia; CDT: Catheter Directed Thrombolysis; CLI: Critical Limb Ischemia; ITT - Intent-to-Treat
1. Introduction
Acute limb ischemia (ALI) is characterized as an abrupt decrease in limb perfusion that compromises the limb's viability [1]. The incidence of the ALI is approximately 1.5 cases per 10,000 persons per year [2]. The 30-day mortality rate for patients with ALI, in the absence of rapid intervention, is approximately 15%, with an amputation rate ranging from 10-30% [3]. Treatment options for ALI include systemic anticoagulation, followed by either open surgical intervention (thromboembolectomy or surgical bypass), or by catheter directed thrombolysis (CDT)) [4]. Various catheter based options include: infusion of fibrinolytic agents (pharmacological thrombo-lysis), pharmacomechanical thrombolysis, catheter-mediated thrombus aspiration, mechanical thrombectomy, and any combination of the above [5].The use of the CDT is an effective option for the treatment of ALI and has been shown to have comparable results to surgical treatment [6-8]. However, there were more bleeding complications in the thrombolysis group [6, 7].
The major complications reported with CDT include major bleeding at the access site or non-access site bleeding, causing early termination of the CDT. Bleeding at the puncture site is more common [8-11]. Currently, there are no devices on the market that are approved and intended for vascular access and protection during CDT in patients critical limb ischemia (CLI). CaveoMed developed the CaveoVasc® Thrombolysis Protection System, a vascular access protection for use in thrombolysis procedures. The function is to minimize risks of access site bleeding complications during lengthy CDT procedures. Pressure balloons are inflated outside the artery to maintain a tight seal of the arterial puncture site during thrombolysis. The aim of the study was to evaluate the safety and effectiveness of the new CaveoVasc® protection system in patients undergoing CDT.
2. Materials and Methods
2.1 Study design
The CaveoVasc® study was a prospective, single-arm study performed at the University Heart Center Freiburg – Bad Krozingen. The aim of the study was to evaluate the safety and effectiveness of the new CaveoVasc® thrombolysis protection system for femoral artery access and protection in patients treated with CDT for limb ischemia. The study was performed in accordance with standard EN ISO 14155 for clinical investigations with medical devices on human subjects and recommendations guiding physicians in biomedical research involving human subjects adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964 and later revisions. The clinical investigational plan, informed consent, any other specific study documents were reviewed and approved by the local Ethics Committee and Competent Authority before enrolment.
2.2 Patient population
Patients, who received CDT due to a CLI were included in this study. Supplement Table 1 shows the inclusion and exclusion criteria.
2.3 Investigational device
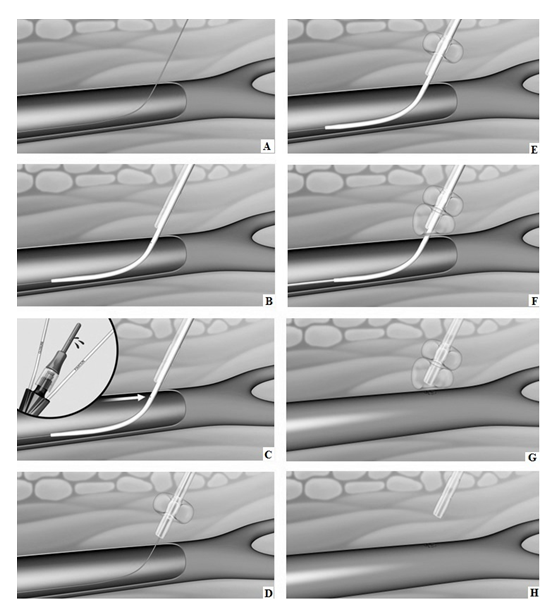
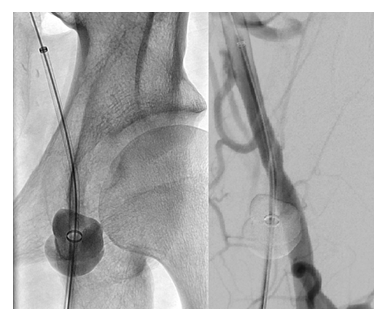
The CaveoVasc® Thrombolysis Protection System is a sterile, single use disposable device which provides vessel access for a CDT catheter and access site protection during thrombolysis. The CaveoVasc® Thrombolysis Protection System is intended to facilitate sheath access, and reduce bleeding complications during CDT, for treatment of limb ischemia, protecting the puncture site by providing sheath stabilization using a double balloon technique, tamponading small vessels in the puncture channel and protecting the access site at the femoral artery. The CaveoVasc® Thrombolysis Protection System is indicated for adult patients with a diagnosis of limb ischemia requiring treatment with CDT. A schematic representation of the use of the CaveoVasc® Thrombolysis Protection System is given in Figure 1. An angiogram after placement of the CaveoVasc® Thrombolysis Protection System is shown in Figure 2. The device got CE Mark June 2020.
2.4 Intervention
A retrograde approach was used except in one intervention where an antegrade approach was chosen. After intraluminal recanalization of the occlusion a 0.018-inch wire was inserted and over this wire the 6F Rotarex STM thrombectomy catheter (Straub Medical AG, Vitters-Wangs, Switzerland) was introduced. The number of passages with the system was decided by the investigator. The decision to induce a thrombolysis was left to the investigator. CDT was delivered using a multisidehole catheter (Cragg McNamara; ev3 Endovascular Inc, Plymouth, Minn or Unifuse; Angiodynamics, Latham, NY). In all cases the agent for thrombolysis was recombinant tissue plasminogen activator. After a bolus was given the flow rate was determined individually. The patients received infusions of heparin controlled by activated partial-thromboplastin time. Patients were monitored in the intensive care unit during lysis therapy in accordance with clinic standards.
Figure 1: Schematic representation of CaveoVasc® procedure. A) Guidewire in femoral artery; B) CaveoVasc® is applied after punction of the femoral artery and placement of the guide wire, thus in the beginning of the procedure; C) Removal of Locator, blood backflow indicates correct position; D) Infaltion of the Fixation Balloon – the inflated balloon secures the position of CaveoVasc® in the tissue; E) Placement of the sheath; F) Inflation of Pressure Balloon – Start of thrombolysis therapy via catheter perfusion; G) Removal of the sheath and catheter after the end of the thrombolysis procedure; H) Removal of CaveoVasc®.
2.5 Study endpoints
The primary safety endpoint was the rate of all major bleeding (BARC > type 3, supplement table 2) from the start to the end of the CDT procedure. Secondary safety endpoints were bleeding (BARC ≥ 1 and BARC ≥ 2) from the start of the CDT procedure to the end of the CDT procedure and adverse events were recorded at discharge and 30 days. The primary performance endpoint was length of CDT. Secondary performance endpoints were the rate of subjects completing the CDT without early interruption due to access site complication, the rate of successful thrombolysis without bleeding complication and pain at access site. Access site pain was evaluated using the verbal pain scale (0 to 10).
2.6 Statistical analysis
The statistical analysis was performed with the SAS System® Version 9.4. The safety population included all patients enrolled in the study. The Intent-to-Treat (ITT) population included all subjects who signed their informed consent with a device implant attempt. The per protocol (PP) population included all subjects from the ITT population without major deviations. Continuous data is presented as means ± standard deviation; categorical data is expressed as counts (percentages).
3. Results
Twenty patients with limb ischaemia requiring treatment with CDT were enrolled. The age ranged between 38 and 88 years (mean 66.2 ± 12.7). Seventeen (85%) of the population were male patients. Cardiovascular risk factors and relevant laboratory parameters are shown in Table 1. All study devices were inserted and removed successfully. No device deficiency was recorded during the study. The lysis catheter could not be inserted in one patient due to anatomical reasons (the target vessel had been ligated proximally after a previous surgical vascular procedure) although the CaveoVasc® Thrombolysis Protection System device was successfully deployed the thrombolysis couldn’t be performed. No major bleeding (BARC > type 3) occurred. Overall the number of minor bleeding episodes during treatment was small. Five bleeding episodes were classified as BARC 1 and BARC 2. Two of these patients had bleeding at the site of the puncture during the procedure.
The average time before catheter removal was 17.32 ± 5.08 hours, and the average time before device removal was 16.98 ± 6.25 hours. The duration of CDT in ITT group and PP group was 16.5 hours ± 5.2 and 16.9 ± 4.7 respectively. Eighteen patients (94.7%) completed the CDT procedure without early interruption. In one patient the CDT procedure failed because of a presumed access site complication due to previous surgical ligation of superficial femoral artery. Pain at the access site was evaluated by the patients at the start of the CDT, after 6 hours and at the end of the CDT procedure. The average pain score was 0.3 ± 1.1, 2.0 ± 2.3 and 0.8 ± 2.0, respectively. In 19 patients a final telephone interview was performed. One death was reported by the family doctor on the 14th post-interventional day due to acute global cardiac decompensation.
|
Factors |
Intent-to-Treat (ITT) n = 20 |
|
N (%) or mean ± SD [min, max] |
|
|
Age (years) |
66.2 ± 12.7 [38, 88] |
|
Male (%) |
17 (85.0) |
|
BMI calculated [kg/m²] |
26.57 ± 4.92 [20.2, 37.9] |
|
Hypertension |
16 (80) |
|
Diabetes mellitus |
6 (30) |
|
Hyperlipidemia |
17 (85) |
|
Smoker |
16 (80) |
|
Coronary heart disease |
9 (45) |
|
Cerebral vascular disease |
2 (10) |
|
Stroke |
3 (15) |
|
Hemoglobin: Value [g/dL] |
13.3 ± 1.9 [8, 16] |
|
RBC: Value [10^12/L] |
4.5 ± 0.7 [3, 6] |
|
WBC: Value |
8236.0 ± 2659.5 [3810, 14730] |
|
Platelets: Value [10^9/L] |
267.7 ± 85.0 [120, 436] |
|
Hematocrit: Value [%] |
39.5 ± 5.4 [26, 48] |
|
Creatinine: Value [mL/min] |
73.6 ± 20.6 [41, 114] |
|
INR: Value |
1.1 ± 0.2 [1, 2] |
BMI- Body mass index, INR – International Normalized Ratio, RBC – Red Blood Cells, WBC - White Blood Cells,
Table 1: Cardiovascular risk factors and relevant laboratory parameters.
4. Discussion
For the treatment of ALI, surgical or endovascular therapies with CDT are available. The results of these treatment options are comparable [6, 7, 8]. In addition to therapy success, most studies evaluate the complications of thrombolytic therapy. Other than acute renal failure and embolization, bleeding complications are the most common [12]. Van den Berg et al. noted that the risk of all complications increased with increasing length of perfusion, from 4% at 8 hours to 34% at 40 hours [9]. Bleeding complications should be differentiated between systemic bleeding and bleeding at the access site. Hemorrhagic stroke and peri-interventional gastrointestinal bleeding are dreaded because they are associated with high mortality [13]. Bleeding at the puncture site is more frequent than systemic bleeding complications [14, 15].
Access site bleeding complications during thrombolytic treatment are reported in almost a quarter of cases [16, 17]. The high rate of complications on the access side can be explained by the long treatment duration. In the present study the lysis time was 17 hours on average. Other studies reported an average lysis time of over 20 hours. [12, 13, 18]. Patients must remain as immobile as possible while the sheath is positioned in the femoral artery. As the duration of treatment increases, it can be presumed that problems at the access site will arise, due to slight movements of the patient. In addition to the vascular trauma caused by the puncture and the indwelling lysis catheter, thrombolysis and the consequent systemic heparinization, causes significant anticoagulation.
The CaveoVasc® Thrombolysis Protection System can be used as add-on to current thrombolysis therapy. It is intended to reduce bleeding complications in CDT by protecting the puncture site by stabilizing the sheath with a double balloon technique and tamponading minor bleeding in the puncture channel. The application is simple and requires only a few additional intervention steps. The feasibility of this approach has been shown and all study devices were inserted and removed successfully. No major bleeding occurred; 5 bleedings classified as BARC 1 and BARC 2 occurred but only two of these patients had bleeding at the puncture site during the procedure. One thrombolysis had to be terminated after a CT scan and angiography were performed for severe abdominal pain and the inflated balloons erroneously interpreted as hematoma and the lysis therapy consequently stopped. However, the patient remained stable and duplex sonography showed no relevant hematoma. In the only patient with an antegrade access a hematoma occurred despite the CaveoVasc® Thrombolysis Protection. The use of the system is now only recommended with retrograde access.
4.1 Limitations
The limitations of this study are the single-arm design and the small sample size. The present study excluded cachectic patients. Further investigation is needed to determine whether the device can be used in very thin patients, as subcutaneous tissue is needed to fix the device. The purpose of the current study was to collect safety and feasibility data for CE marking. The next step should be a randomized controlled trial compared to CDT with and without CaveoVasc® Protection.
5. Conclusion
The study set out to explore the safety and performance of the CaveoVasc® Thrombolysis Protection System in patients treated with CDT for limb ischemia. The results support the conclusion that the device performed as intended as both the primary and secondary endpoints were achieved without major difficulties or adverse events related to the device during the procedure and subsequent follow up.
Acknowledgements
Disclosures
Elias Noory: Honoraria received from: Abbott Vascular, Boston Scientific Corp., Bard BD Interventional, Cardinal Health, Medtronic, Shockwave Medical
Thomas Zeller: Honoraria received from: Abbott Vascular, Veryan, Biotronik, Boston Scientific Corp., Cook Medical, Gore & Associates, Medtronic, Philips-Spectranetics, Shockwave. Consulted for: Boston Scientific Corp., Gore & Associates, Medtronic, Veryan, Intact Vascular, Shockwave, Bayer, Vesper Medical. Research, clinical trial, or drug study funds received from (institution): 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Medtronic, Philips, Terumo, TriReme, Shockwave, Med Alliance, Intact Vascular, B. Braun. Common stock: QT Medical
- The study was presented at the virtual ESVS Translational Meeting June 2020 and the virtual CIRSE Summit September 2020
- This study was supported by CaveoMed GmbH, Tübingen, Germany
Conflict of Interest
The authors have no conflict of interest.
References
- Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, Fowkes F. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Surg 33 (2007): S1-S75.
- Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med 366 (2012): 2198-2206.
- Rajan DK, Patel NH, Valji K, Cardella JF, Brown DB, Brountzos EN, et al. Quality improvement guidelines for percutaneous management of acute limb ischemia. Journal of Vascular and Interventional Radiology 20 (2009): S208-S218.
- Baril D T, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg 60 (2014): 669.e2-677.e2.
- Karnabatidis D, Spiliopoulos S, Tsetis D, Siablis D. Quality Improvement Guidelines for Percutaneous Catheter-Directed Intra-Arterial Thrombolysis and Mechanical Thrombectomy for Acute Lower-Limb Ischemia, Cardiovasc. Intervent. Radiol 34 (2011): 1123-1136.
- Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg 220 (1994): 251-266.
- Ouriel K, Shortell CK, DeWeese JA, Green R M, Francis C W, Azodo M V, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg 19 (1994): 1021-1030.
- Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med 338 (1998): 1105-1111.
- van den Berg J C. Thrombolysis for acute arterial occlusion. Journal of vascular surgery 52 (2010): 512-515.
- Ouriel K, Veith F J, Sasahara A A. Throm-bolysis or peripheral arterial surgery: phase I results. Journal of vascular surgery 23 (1996): 64-75.
- Plate G, Jansson I, Forssell C, Weber P, Oredsson S. Thrombolysis for acute lower limb ischaemia—a prospective, randomised, multicentre study comparing two strategies. European journal of vascular and endovascular surgery 31 (2006): 651-660.
- Byrne R M, Taha A G, Avgerinos E, Marone L K, Makaroun M S, Chaer R A. Contemporary outcomes of endovascular interventions for acute limb ischemia. Journal of Vascular Surgery 59 (2014): 988-995.
- Agle S C, McNally M M, Powell C S, Bogey W M, Parker F M, Stoner M C. The association of periprocedural hypertension and adverse outcomes in patients undergoing catheter-directed thrombolysis. Annals of vascular surgery 24 (2010): 609-614.
- Falkowski A, Poncyljusz W, Samad R A, Mokrzynski S. Safety and efficacy of ultra-high-dose, short-term thrombolysis with rt-PA for acute lower limb ischemia. European Journal of Vascular and Endovascular Surgery 46 (2013): 118-123.
- Nehler M R, Mueller R J, McLafferty R B, Johnson S P, Nussbaum J D, Mattos M A, et al. Outcome of catheter-directed thrombolysis for lower extremity arterial bypass occlusion. Journal of vascular surgery, 37 (2003): 72-78.
- Kuhn J P, Hoene A, Miertsch M, Traeger T, Langner S, Hosten N, et al. Intraarterial recombinant tissue plasminogen activator thrombolysis of acute and semiacute lower limb arterial occlusion: quality assurance, complication management, and 12-month follow-up reinterventions. American Journal of Roentgenology 196 (2011): 1189-1193.
- Korn P, Khilnani N M, Fellers J C, Lee T Y, Winchester P A, Bush H L, et al. Thrombolysis for native arterial occlusions of the lower extremities: clinical outcome and cost. Journal of vascular surgery 33 (2001): 1148-1157.
- Grip O, Kuoppala M, Acosta S, Wanhainen A, Åkeson J, Björck M. Outcome and complications after intra-arterial thrombolysis for lower limb ischaemia with or without continuous heparin infusion. The British journal of surgery 101 (2014): 1105-1119.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 123 (2011): 2736-2747.
Supplementary Information
|
Inclusion Criteria |
|
Age ≥18 years |
|
Diagnosis of limb ischemia requiring CDT |
|
Patient understands and signs the study specific written informed consent form |
|
Patient presents with indications for catheter-guided thrombolysis therapy, per the hospital standards, and in accordance with current guidelines. |
|
Exclusion Criteria |
|
Patients who are currently participating in another clinical trial of an investigational drug or device that has not concluded the follow-up period |
|
Patients who cannot adhere to or complete the investigational protocol for any reason |
|
Inability to provide informed consent or to comply with study assessments (e.g. due to cognitive impairment or geographic distance) |
|
Patients with bleeding disorders such as thrombocytopenia (platelet count<100,000/mm3), hemophilia, von Willebrand’sdisease or anemia(Hgb<10g/ dL, Hct< 30%) |
|
Patients who need a puncture needle longer than 8 cm due to morbid obesity |
|
Patients who are cachectic and do not have enough subcutaneous tissue/fat to accommodate the CaveoMed device (the two balloons, with 2 ml of contrast-enriched saline in each ballon) |
|
Patients with aneurysms, dissections or contortions in the femoral vessels |
|
Patients with amputations on the ipsilateral or contralateral let |
|
Patients who have had a percutaneous intervention through the leg vessels within the past 30 days before enrollmentin the study |
|
Patients who have already had a closure system implanted in their leg arteries, that is permanent, and not manufactured from material that resorbs by the body after a period of time |
|
Serious concomitant disease with an anticipated life expectancy less than 12 months |
|
Uncontrollable systemic hypertension |
|
Active systemic or cutaneous infection or inflammation |
|
Patients who are pregnant or lactating |
|
Patients with documented INR > 1.5 or patients currently receiving glycoprotein IIb/IIIaplatelet inhibitors, unless the glycoprotein IIb/IIIaplatelet inhibitor is given as a bolus prior to the CDT as part of the institution’s standard of care |
|
Intercranialhemorrage |
|
Onset of compartment syndrome |
|
Severe lower limb ischemia that requires immediate surgical intervention |
|
Prior, recent (within 3 months) abdominal or lower limb vascular surgery or non-vascular surgery procedure |
Table 1
|
Bleeding Academic Research Consortium Definition for Bleeding [19] |
|
Type 0: no bleeding |
|
Type 1: bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a healthcare professional, may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a healthcare professional |
|
Type 2: any overt, actionable sign of hemorrhage (eg. more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that not fit the criteria for type 3,4 or 5 but does meet at least one of the following criteria (1) requiring nonsurgical, medical intervention by a healthcare professional, (2) leading to hospitalization or increased level of care, or (3) prompting evaluation |
|
Type3 |
|
Type 3 a: Overt bleeding plus hemoglobin drop of 3 to < 5g/dl (provided hemoglobin drop is related to bleed), any transfusion with overt bleeding |
|
Type 3b: Overt bleeding plus hemoglobin drop >5 g/dL* (provided hemoglobin drop is related to bleed), cardiac tamponade, bleeding requiring surgical intervention for control (excluding dental/nasal/skin/hemorrhoid), bleeding requiring intravenous vasoactive agents. |
|
Type 3c: Intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation, does include intraspinal), subcategories confirmed by autopsy or imaging or lumbar puncture, intraocular bleed compromising vision. |
|
Type 4: CABG-related bleeding (not applicable for this study) |
|
Type 5 |
|
Type 5a: Probable fatal bleeding; no autopsy or imaging confirmation but clinically suspicious |
|
Type 5b: Definite fatal bleeding; overt bleeding or autopsy or imaging confirmation. |
Table 2